1191237-69-0
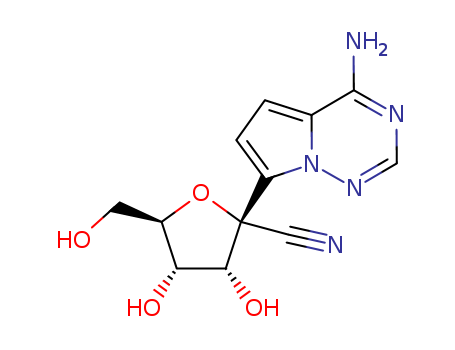
- Product Name:GS441524
- Molecular Formula:
- Purity:99%
- Molecular Weight:291.266
Product Details;
CasNo: 1191237-69-0
supply 99.9% High Quality GS441524 1191237-69-0 with low price
- Molecular Formula:C12H13N5O4
- Molecular Weight:291.266
- Melting Point:> 233° C (dec.)
- Density:1.84±0.1 g/cm3(Predicted)
GS441524 (Cas 1191237-69-0) Usage
|
Description |
GS-441524 is a nucleoside analogue that terminates the RNA chain of viral RNA-dependent RNA polymerase. GS-441524 was developed by Gilead Sciences, Inc as part of their research into human RNA virus disease such as Ebola. GS-441524 is a small molecule that exhibits potent antiviral activity against a number of RNA viruses, including the zoonotic severe acute respiratory syndrome (SARS) coronavirus ( Cho et al., 2012). A phosphoramidate prodrug of GS-441524 (GS-5734) has been previously shown to inhibit the replication of several taxonomically diverse RNA viruses such as Middle East respiratory syndrome virus, Ebola virus, Lassa fever virus, Junin virus and respiratory syncytial virus, while having low cytotoxicity in a wide-range of cell lines ( Sheahan et al., 2017 ). GS-5734 has also been shown to protect rhesus monkeys from experimental Ebola virus infection ( Warren et al., 2016 ). |
|
Mechanism of action |
GS-441524 requires intracellular phosphorylation via cellular kinases to a nucleoside monophosphate and subsequently to the active triphosphate metabolite (NTP) ( Cho et al., 2012 ; Sheahan et al., 2017; Warren et al., 2016). The active NTP analog functions as a competitor of the natural nucleoside triphosphates in viral RNA synthesis. The active form of GS-441524 has been shown to inhibit RSV RNA-dependent RNA polymerase mediated transcription by incorporating into the nascent viral transcript and causing premature termination ( Sheahan et al., 2017 ). We hypothesized that GS-441524 would be activated in feline cells, attenuate FIPV replication, have low cytotoxicity in feline cells in vitro and effectively treat cats with experimentally induced FIP. |
|
Pharmacokinetics |
A pharmacokinetic (PK) study was performed in laboratory cats to determine the metabolism and acute animal toxicity of GS-441524. GS-441524 was dissolved at a concentration of 12.5 mg/ml in 5% ethanol, 30% propylene glycol, 45% PEG 400, 20% water, and adjusted to pH 1.9 with concentrated HCl. Six laboratory cats were randomly divided into group A (n = 3; IV administration) or B (n = 3; SC administration). At time point zero, Group A cats were administered 5 mg compound/kg body weight intravenously while Group B cats received 5 mg com- pound/kg subcutaneously. Cats were then monitored for signs of acute toxicity (elevated pulse, respiratory distress, cyanosis, diarrhea, anorexia, drooling, vomiting, ataxia, weight loss, and changes in rectal temperature) daily for five days. Serial 0.5 ml whole blood samples in EDTA were obtained by lateral saphenous, superficial brachial or jugular venipuncture from each cat at 0.25, 0.5, 1, 2, 4, 6, 8, 12 and 24 h. After collection, blood samples were immediately placed onice and then centrifuged at 5000 rpm for 5 min. The isolated plasma was pipetted into a 1.5 ml microcentrifuge tube and frozen at ? 70 °C for further analysis of free GS-441524. The buffy coat fraction from each blood collection was suspended in 1.5 ml phosphate free Tris-buffered saline (TBS, 50 mM Tris-Cl, pH 7.5, 150 mM NaCl) and PBMC isolated by Ficoll Hypaque density gradient centrifugation. The plasma and PBMC fractions were snap frozen in liquid nitrogen and shipped on dry ice to Gilead Sciences, Inc. (Foster City, CA) for further analyses. (plasma drug concentration and intracellular phosphorylation analyses). |
|
Uses |
GS-441524 is originally identified as an active metabolite of Remdesivir that is an antiviral drug, a novel nucleotide analog prodrug. |
|
Acquired resistance |
Resistance to GS-441524 can exist at the time of diagnosis, but this is uncommon. Rather, it tends to occur during treatment and is often partial at first and necessitates a higher dosage to accommodate for it. It can become total in some cats. Resistance is the biggest problem in cats with neurological disease, especially those that present with neurological disease or develop brain infections during treatment or during a relapse after what appears to have been a successful treatment. Many cats with partial drug resistance can be treated for their disease signs but will relapse as soon as the treatment is stopped. There have been cats "treated" for FIP for over a year with no cure, but ultimately resistance becomes worse or the owner runs out of money. |
|
Biological Activity |
GS 441524 is a viral RNA-dependent RNA polymerase (RdRp) inhibitor and broad spectrum antiviral nucleotide; active metabolite of Remdesivir. Competes with natural nucleoside triphosphates, blocking viral RNA synthesis. Exhibits antiviral activity against viruses from Coronaviridae family including SARS-CoV-2 in vitro, and SARS-CoV and feline infectious peritonitis virus (FIPV) in vitro and in vivo. Inhibits SARS-CoV and MERS-CoV- infected HAE cultures (EC50 values are 0.18 and 0.86 μM, respectively) and inhibits murine hepatitis virus (MHV) (EC50 = 1.1 μM). |
|
Side effects |
GS-441524 treatment is amazingly free of systemic side effects. It can cause minor kidney damage in some cats, but this does not progress to overt renal disease. Systemic drug reactions of the vasculitis type have been seen in a few cats and can be confused with injection site reactions. However, these drug reactions are at non-injection sites and are often self-limiting or respond well to a short-term low dose of steroids. The major side-effect of GS treatment is pain at the injection sites, which varies from cat to cat and according to the injection prowess of the person doing the treatment (usually the owner). Injection site sores are a problem with some owners and usually occur when the injection site is not moved around the body (stay away from between the shoulders) and not given into the muscle and nerve layers below the subcutis. I recommend selecting sites starting an inch behind the shoulder blades, down the back to 1-2 inches before the tailhead, and one third to one-half of the way down the chest and abdomen. Many people use gabapentin before injections to help ease the pain. Injection site sores are cleared of surrounding hair and gently cleaned 4 or more times a day with sterile cotton balls soaked in 1:5 dilution of household hydrogen peroxide. They usually do not require any more sophisticated treatment and heal within 2 weeks or so. |
|
Preparation and handling |
GS-441524 is supplied as a solid. A stock solution may be made by dissolving the GS-441524 in the solvent of choice, which should be purged with an inert gas. GS-441524 is soluble in organic solvents such as DMSO and dimethyl formamide. The solubility of GS-441524 in these solvents is approximately 10 mg/ml. GS-441524 is sparingly soluble in aqueous buffers. For maximum solubility in aqueous buffers, GS-441524 should first be dissolved in DMSO and then diluted with the aqueous buffer of choice. GS-441524 has a solubility of approximately 0.16 mg/ml in a 1:5 solution of DMSO:PBS (pH 7.2) using this method. We do not recommend storing the aqueous solution for more than one day. |
|
Consumer Uses |
ECHA has no public registered data indicating whether or in which chemical products the substance might be used. ECHA has no public registered data on the routes by which this substance is most likely to be released to the environment. |
1191237-69-0 Relevant articles
Synthesis method of antiviral drug ridexivir and intermediate thereof
-
, (2021/02/06)
The invention discloses a synthesis meth...
Method for preparing retegravir by using micro-channel reactor
-
, (2021/04/21)
The invention discloses a method for syn...
Synthesis method of key intermediate of ridecevir
-
, (2021/08/07)
The invention provides a novel method fo...
METHODS OF PREPARING 1'-CYANO NUCLEOSIDES
-
, (2021/09/17)
The present disclosure generally describ...
1191237-69-0 Process route
-
![(2R,3R,4R,5R)‐2‐{4‐aminopyrrolo[2,1‐f][1,2,4]triazin‐7‐yl}‐3,4‐bis(benzyloxy)‐5‐[(benzyloxy)methyl]tetrahydrofuran‐2‐carbonitrile](/upload/2023/8/8b0772c1-6284-4a85-95a0-935e20676d6d.png)
- 1355357-49-1
(2R,3R,4R,5R)‐2‐{4‐aminopyrrolo[2,1‐f][1,2,4]triazin‐7‐yl}‐3,4‐bis(benzyloxy)‐5‐[(benzyloxy)methyl]tetrahydrofuran‐2‐carbonitrile

-
![(2R,3R,4S,5R)?2?(4?aminopyrrolo[1,2?f][1,2,4]triazin?7?yl)?3,4?dihydroxy?5?(hydroxymethyl)tetrahydrofuran?2?carbonitrile](/upload/2023/8/f051962e-4af6-4b0f-8b38-e02430149248.png)
- 1191237-69-0
(2R,3R,4S,5R)?2?(4?aminopyrrolo[1,2?f][1,2,4]triazin?7?yl)?3,4?dihydroxy?5?(hydroxymethyl)tetrahydrofuran?2?carbonitrile
| Conditions | Yield |
|---|---|
|
With boron trichloride; In dichloromethane; at 5 ℃;
|
95% |
|
With 5%-palladium/activated carbon; hydrogen; In ethanol; at 45 - 50 ℃; under 750.075 - 900.09 Torr; Solvent; Reagent/catalyst;
|
91.1% |
|
(2R,3R,4R,5R)‐2‐{4‐aminopyrrolo[2,1‐f][1,2,4]triazin‐7‐yl}‐3,4‐bis(benzyloxy)‐5‐[(benzyloxy)methyl]tetrahydrofuran‐2‐carbonitrile; With boron trichloride; In dichloromethane; at -78 - -40 ℃; for 2h; Inert atmosphere;
With methanol; triethylamine; In dichloromethane;
|
85% |
|
With methanol; boron trichloride; triethylamine; In dichloromethane; for 0.0433333h; Temperature; Flow reactor;
|
82% |
|
With boron trichloride; In dichloromethane; at -78 ℃; Inert atmosphere;
|
70% |
|
With boron trichloride; In dichloromethane; at -20 - -15 ℃; for 1h;
|
|
|
Multi-step reaction with 2 steps
1.1: boron trichloride; Trimethyl borate / dichloromethane / 2 h / 0 - 35 °C
1.2: 12 h / 20 - 25 °C
2.1: potassium carbonate / water / 1 h / 20 °C
With Trimethyl borate; boron trichloride; potassium carbonate; In dichloromethane; water;
|
-
![(3R,4R,5R)-2-(4-aminopyrrolo[2,1-f][1,2,4]triazin-7-yl)-3,4-bis(benzyloxy)-5-((benzyloxy) methyl)tetrahydrofuran-2-carbonitrile](/upload/2023/8/b7080baf-0005-43e2-a5ed-9c661ee47a6b.png)
- 1191237-68-9
(3R,4R,5R)-2-(4-aminopyrrolo[2,1-f][1,2,4]triazin-7-yl)-3,4-bis(benzyloxy)-5-((benzyloxy) methyl)tetrahydrofuran-2-carbonitrile

-
![(2R,3R,4S,5R)?2?(4?aminopyrrolo[1,2?f][1,2,4]triazin?7?yl)?3,4?dihydroxy?5?(hydroxymethyl)tetrahydrofuran?2?carbonitrile](/upload/2023/8/f051962e-4af6-4b0f-8b38-e02430149248.png)
- 1191237-69-0
(2R,3R,4S,5R)?2?(4?aminopyrrolo[1,2?f][1,2,4]triazin?7?yl)?3,4?dihydroxy?5?(hydroxymethyl)tetrahydrofuran?2?carbonitrile

-
![(2S,3R,4S,5R)-2-(4-aminopyrrolo[2,1-f][1,2,4]triazin-7-yl)-3,4-dihydroxy-5-(hydroxymethyl)tetrahydrofuran-2-carbonitrile](/upload/2023/8/fe03c1f6-b6f3-4b96-a4f9-0c1da35f1e96.png)
- 1355049-95-4
(2S,3R,4S,5R)-2-(4-aminopyrrolo[2,1-f][1,2,4]triazin-7-yl)-3,4-dihydroxy-5-(hydroxymethyl)tetrahydrofuran-2-carbonitrile
| Conditions | Yield |
|---|---|
|
(3R,4R,5R)-2-(4-aminopyrrolo[2,1-f][1,2,4]triazin-7-yl)-3,4-bis(benzyloxy)-5-((benzyloxy) methyl)tetrahydrofuran-2-carbonitrile; With boron trichloride; In dichloromethane; at -78 ℃; for 1h; Inert atmosphere;
With methanol; In dichloromethane;
|
37% 37% |
|
With boron trichloride; In dichloromethane; at -78 ℃; for 1h; Inert atmosphere;
|
37% 37% |
|
With boron trichloride; In dichloromethane; at -78 - 0 ℃; for 3h;
|
6% 22% |
|
In dichloromethane; at -78 ℃; for 1h;
|
|
|
With boron trichloride; In dichloromethane; at -78 - -20 ℃; for 1h; Inert atmosphere;
|
1191237-69-0 Upstream products
-
14233-64-8

(3R,4R,5R)-3,4-Bis-benzyloxy-5-benzyloxymethyl-dihydro-furan-2-one
-
16838-89-4
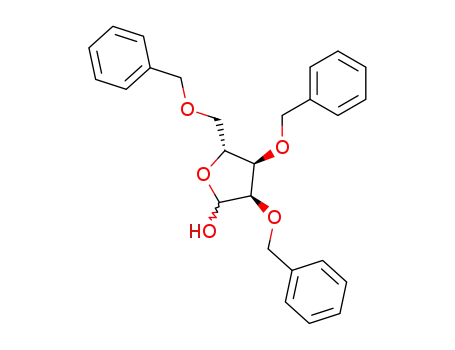
2,3,5-tri-O-benzyl-D-ribofuranose
-
1191237-68-9

(3R,4R,5R)-2-(4-aminopyrrolo[2,1-f][1,2,4]triazin-7-yl)-3,4-bis(benzyloxy)-5-((benzyloxy) methyl)tetrahydrofuran-2-carbonitrile
-
1355049-94-3
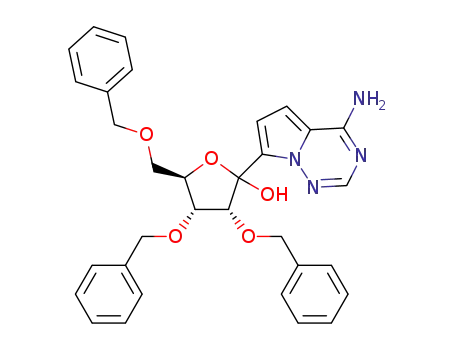
(3R,4R,5R)-2-(4-aminopyrrole-[2,1-f][1,2,4]-triazin-7-yl)-3,4-bis(benzyloxy)-5-((benzyloxy)methyl)tetrahydrofuran-2-ol
1191237-69-0 Downstream products
-
1355050-10-0
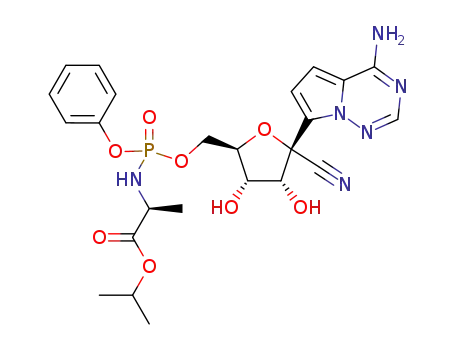
(2S)-isopropyl 2-(((((2R,3S,4R,5R)-5-(4-aminopyrrolo[2,1-f][1,2,4]triazin-7-yl)-5-cyano-3,4-dihydroxytetrahydrofuran-2-yl)methoxy)(phenoxy)phosphoryl)amino)propanoate
-
1809249-37-3
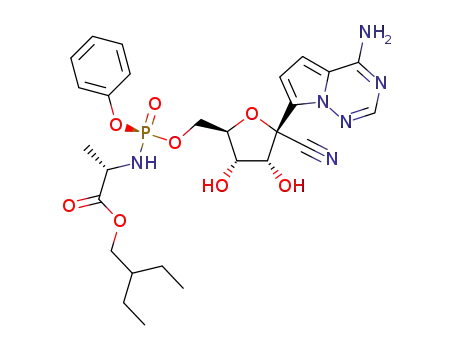
(2S)-2-ethylbutyl 2-(((S)-(((2R,3S,4R,5R)-5-(4-aminopyrrolo[2,1-f][1,2,4]triazin-7-yl)-5-cyano-3,4-dihydroxytetrahydrofuran-2-yl)methoxy)(phenoxy)phosphoryl)amino)propanoate
-
1191237-80-5
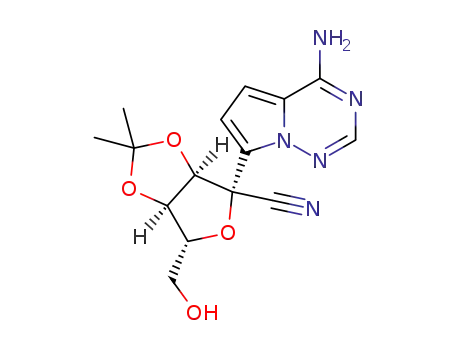
(3αR,4R,6R,6αR)-4-(4-aminopyrrolo[2,1-f][1,2,4]triazin-7-yl)-6-(hydroxymethyl)-2,2-dimethyltetrahydrofuro[3,4-d][1,3]dioxole-4-carbonitrile
-
1911578-75-0
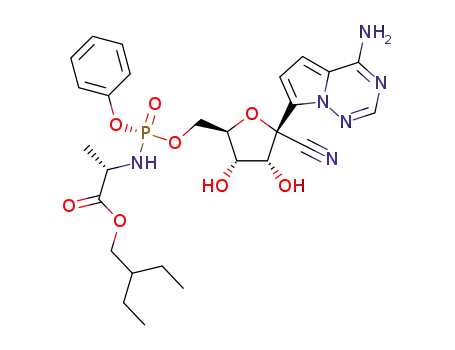
(2S)-2-ethylbutyl 2-(((R)-(((2R,3S,4R,5R)-5-(4-aminopyrrolo[2,1-f][1,2,4]triazin-7-yl)-5-cyano-3,4-dihydroxytetrahydrofuran-2-yl)methoxy)(phenoxy)phosphoryl)amino)propanoate
Relevant Products
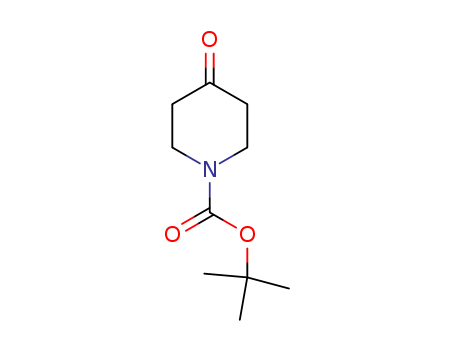
-
N-(tert-Butoxycarbonyl)-4-piperidone
CAS:79099-07-3
-
turkesterone
CAS:41451-87-0
-
Lidocaine hydrochloride
CAS:73-78-9
-
Hyaluronic acid
CAS:9004-61-9