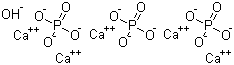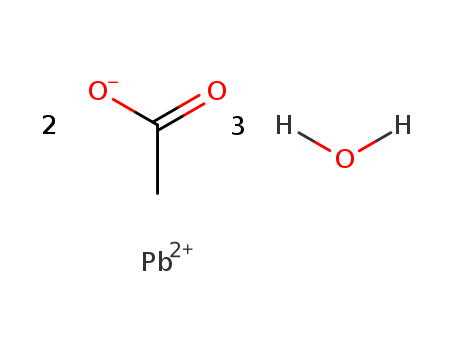
6080-56-4
- Product Name:Lead acetate trihydrate
- Molecular Formula:C4H6O4Pb.3H2O
- Purity:99%
- Molecular Weight:379.335
Product Details;
CasNo: 6080-56-4
Molecular Formula: C4H6O4Pb.3H2O
Appearance: White crystals
Good Manufacturer Fast Delivery Lead acetate trihydrate 6080-56-4 In Stock
- Molecular Formula:C4H6O4Pb.3H2O
- Molecular Weight:379.335
- Appearance/Colour:White crystals
- Vapor Pressure:13.9mmHg at 25°C
- Melting Point:75 °C (dec.)(lit.)
- Boiling Point:117.1 °C at 760 mmHg
- Flash Point:40 °C
- PSA:80.29000
- Density:2,55 g/cm3
- LogP:-0.54610
Lead acetate trihydrate(Cas 6080-56-4) Usage
|
Chemical Properties |
White Crystals |
|
Uses |
Lead(II) acetate trihydrate is used as a mordant in textile printing and dyeing, drier in paints, varnishes and as a water repellant. It is also used in cosmetics and to prepare other lead compounds. It serves as an ingredient in progressive types of hair coloring dyes. Further, it is used in the detection of poisonous gas hydrogen sulfide. |
|
General Description |
White powder or white chunky solid. Slight odor. pH (5% aqueous solution at 77°F) 5.5-6.5. |
|
Air & Water Reactions |
Slowly effloresces in air. Water soluble but solutions absorb carbon dioxide from the air and to give incompletely soluble products. |
|
Reactivity Profile |
Lead acetate trihydrate may react with acids and oxidizing agents. Is incompatible with sulfates, citrates, tartrates, chlorides, carbonates, alkalis, tannin, resorcinol, salicylic acid, phenol, chloral hydrate, sulfites, vegetable infusions and tinctures. Incompatible with phosphates . Incompatible with KBrO3 and with EDTA. |
|
Fire Hazard |
Literature sources indicate that Lead acetate trihydrate is nonflammable. |
|
Purification Methods |
Crystallise it twice from anhydrous acetic acid and dry it under vacuum for 24hours at 100o. The solubility in H2O is 63% (at ~20o) and 200% (at boiling point). [Beilstein 2 IV 118.] |
InChI:InChI=1/2C2H4O2.3H2O.Pb/c2*1-2(3)4;;;;/h2*1H3,(H,3,4);3*1H2;
6080-56-4 Process route
-
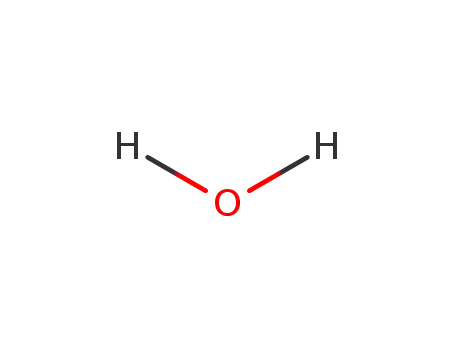
-
7732-18-5
water

-
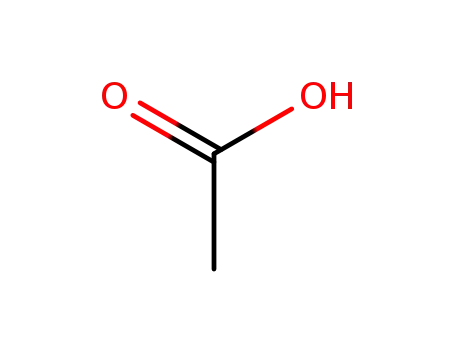
-
64-19-7,77671-22-8
acetic acid

-
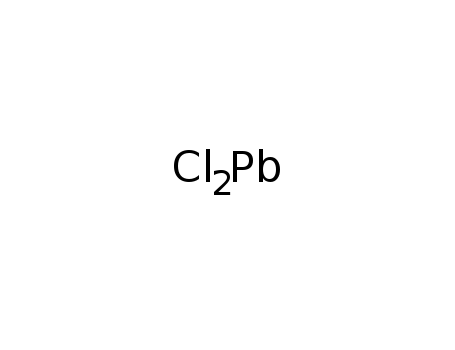
-
7758-95-4
lead(II) chloride

-
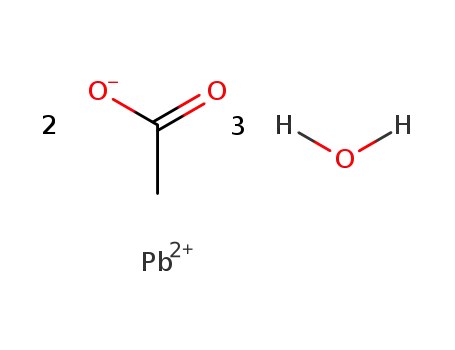
-
6080-56-4
lead(II) acetate trihydrate
| Conditions | Yield |
|---|---|
|
With
sodium carbonate;
In
acetic acid;
preparation of PbCO3 by treatment of PbCl2 with 1.5fold excess Na2CO3, treatment with acetic acid;;
|
86.5% |
|
With
Na2CO3;
In
acetic acid;
aq. acetic acid; preparation of PbCO3 by treatment of PbCl2 with 1.5fold excess Na2CO3, treatment with acetic acid;;
|
86.5% |
-
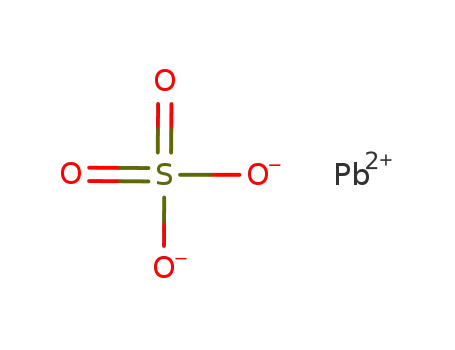
-
lead(II) sulfate

-

-
7732-18-5
water

-

-
64-19-7,77671-22-8
acetic acid

-

-
6080-56-4
lead(II) acetate trihydrate
| Conditions | Yield |
|---|---|
|
With
sodium hydroxide;
In
acetic acid;
separation of sulfate with hot NaOH, react. with acetic acid;;
|
|
|
With
NaOH;
In
acetic acid;
aq. acetic acid; separation of sulfate with hot NaOH, react. with acetic acid;;
|
6080-56-4 Upstream products
-
546-67-8
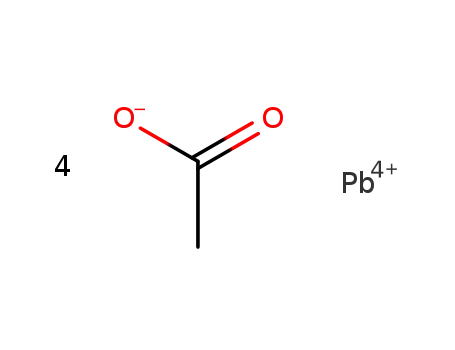
lead(IV) tetraacetate
-
64-19-7

acetic acid
-
7732-18-5

water
-
141-78-6
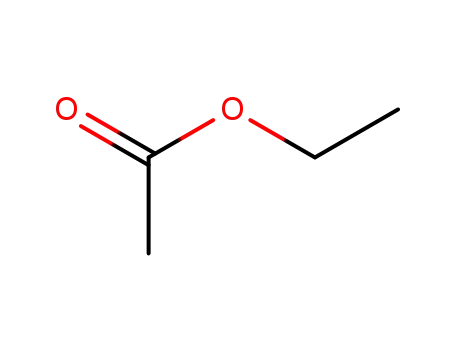
ethyl acetate
6080-56-4 Downstream products
-
6598-47-6
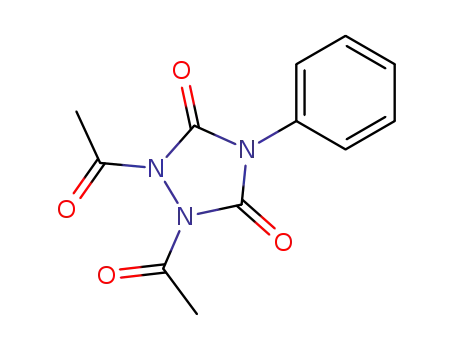
1,2-diacetyl-4-phenyl-1,2,4-triazolidine-3,5-dione
-
546-67-8

lead(IV) tetraacetate
-
131678-11-0
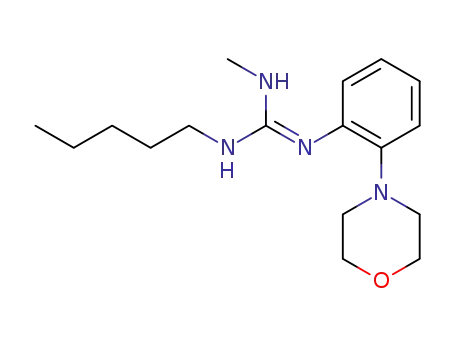
1-methyl-2-(2-morpholinophenyl)-3-(n-pentyl)guanidine
-
131679-64-6
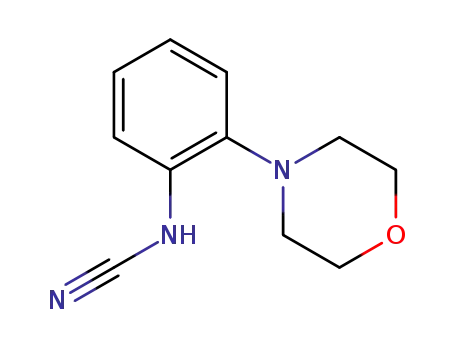
N-(2-morpholinophenyl)cyanamide
Relevant Products
-
Hydroxyapatite
CAS:1306-06-5
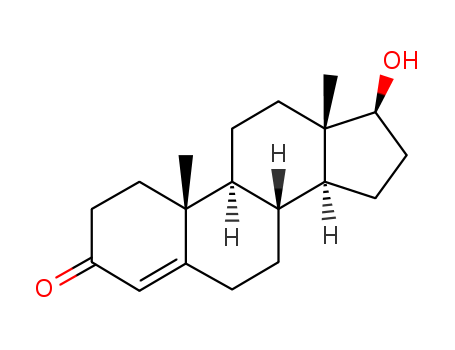
-
Testosterone
CAS:58-22-0
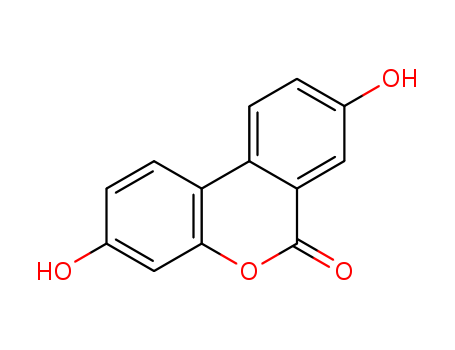
-
3,8-dihydroxy-6H-dibenzo(b,d)pyran-6-one
CAS:1143-70-0