532-24-1
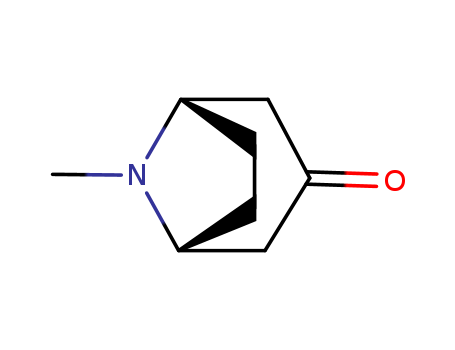
- Product Name:Tropinone
- Molecular Formula:C8H13NO
- Purity:99%
- Molecular Weight:139.197
Product Details;
CasNo: 532-24-1
Molecular Formula: C8H13NO
Appearance: white to light yellow crystal powder
Hot Sale low price Best Quality Tropinone 532-24-1
- Molecular Formula:C8H13NO
- Molecular Weight:139.197
- Appearance/Colour:white to light yellow crystal powder
- Vapor Pressure:0.135mmHg at 25°C
- Melting Point:40-44 °C(lit.)
- Refractive Index:1.4598 (estimate)
- Boiling Point:217.1 °C at 760 mmHg
- PKA:8.93±0.20(Predicted)
- Flash Point:90 °C
- PSA:20.31000
- Density:1.066 g/cm3
- LogP:0.75000
Tropinone(Cas 532-24-1) Usage
|
Chemical Properties |
Tropinone is a white to light yellow crystal powder, It is an alkaloid and a precursor to a number of additional plant alkaloids. Tropinone is the first intermediate in the biosynthesis of the pharmacologically important tropane alkaloids that possesses the 8-azabicyclo[3.2.1]octane core bicyclic structure that defines this alkaloid class. Chemical synthesis of tropinone was achieved in 1901 but the mechanism of tropinone biosynthesis has remained elusive. |
|
Uses |
Tropinone is an oxidative product of Tropane, used to synthesize Morphine and Tropane alkaloids. |
|
Preparation |
The first synthesis of tropinone was by Richard Willst?tter in 1901. It started from the seemingly related cycloheptanone, but required many steps and had an overall yield of only 0.75%.Willst?tter had previously synthesized cocaine from tropinone, in what was the first synthesis and elucidation of the structure of cocaine. The 1917 synthesis by Robinson is considered a legend in total synthesis due to its simplicity and biomimetic approach. Tropinone is a bicyclic molecule, but the reactants used in its preparation are fairly simple: succinaldehyde, methyl amine and acetone dicarboxylic acid (or even acetone). The synthesis is a good example of a biomimetic reaction or biogenetic-type synthesis because biosynthesis makes use of the same building blocks. It also demonstrates a tandem reaction in a one-pot synthesis. Furthermore the yield of the synthesis was 17% and with subsequent improvements exceeded 90%. |
InChI:InChI=1/C8H13NO/c1-9-6-2-3-7(9)5-8(10)4-6/h6-7H,2-5H2,1H3/p+1/t6-,7+
532-24-1 Relevant articles
-
Polievktov et al.
, (1975)
-
-
Glushkov,R.G. et al.
, (1975)
-
Bioactivity-guided synthesis of tropine derivatives as new agonists for melatonin receptors
Yin, Xiu-Juan,Geng, Chang-An,Huang, Xiao-Yan,Chen, Hao,Ma, Yun-Bao,Chen, Xing-Long,Sun, Chang-Li,Yang, Tong-Hua,Zhou, Jun,Zhang, Xue-Mei,Chen, Ji-Jun
, p. 45059 - 45063 (2016)
Twenty-three tropine derivatives as new ...
METHOD OF PRODUCING TERTIARY AMINE OR TERTIARY AMINE DERIVATIVE
-
Paragraph 0066; 0073; 0079, (2018/10/31)
PROBLEM TO BE SOLVED: To provide a metho...
N-Methylation of Amines with Methanol at Room Temperature
Tsarev, Vasily N.,Morioka, Yuna,Caner, Joaquim,Wang, Qing,Ushimaru, Richiro,Kudo, Akihiko,Naka, Hiroshi,Saito, Susumu
supporting information, p. 2530 - 2533 (2015/05/27)
N-Methylation of amines with methanol pr...
A facile synthesis of enantiopure 7-benzyloxycarbonyl-7-azabicyclo [2.2.1]heptane-2-carboxylic acid
Chiou, Wen-Hua,Chiang, Yu-Min,Chen, Guei-Tang
, p. 92 - 97 (2014/02/14)
An efficient procedure for the preparati...
532-24-1 Process route
-
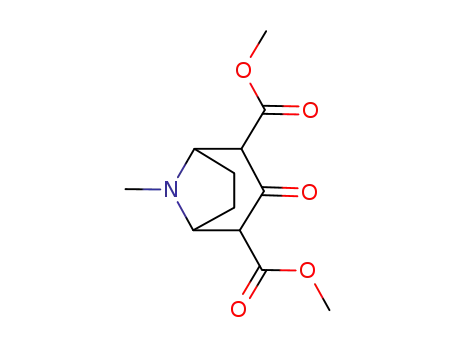
-
100371-46-8
3-oxo-tropane-2,4-dicarboxylic acid dimethyl ester

-
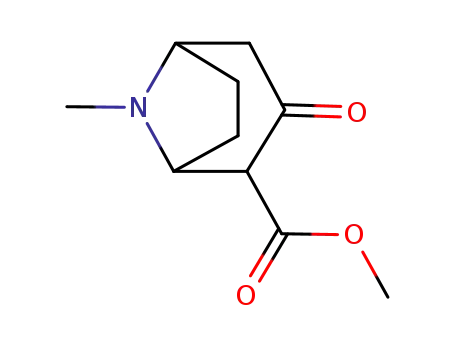
-
36127-17-0
(+/-)-2-(carbomethoxy)-3-tropinone

-
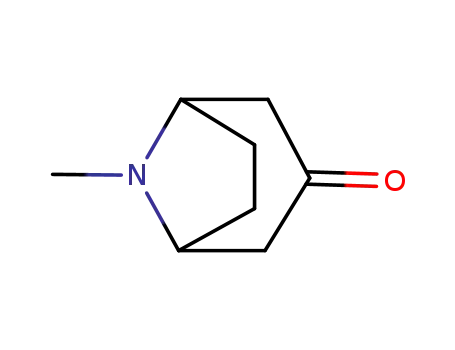
-
532-24-1
tropinone
| Conditions | Yield |
|---|---|
|
With
phosphate buffer;
In
water;
at 20 ℃;
for 24h;
pH=8.0;
|
55% 13% |
-
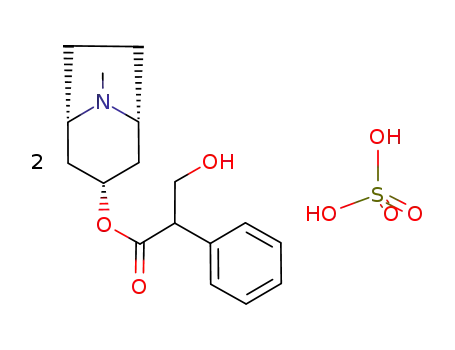
-
55-48-1,300-40-3,620-61-1,1867-24-9,2472-17-5,2623-69-0,14844-27-0,5908-99-6
(1R,3r,5S)-3-tropoyloxytropanium sulfate

-
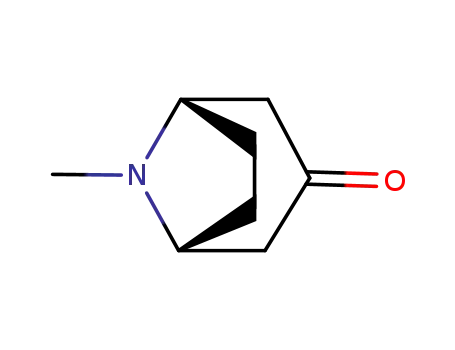
-
532-24-1
tropinone

-
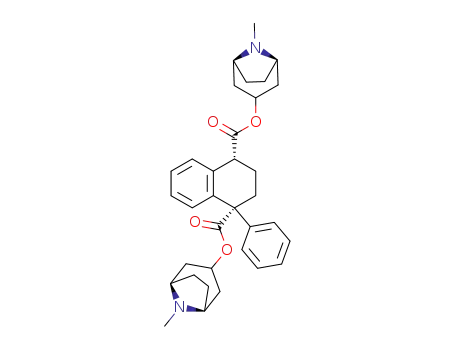
-
510-25-8,5878-33-1,6696-63-5,23852-32-6
β-Belladonnin

-
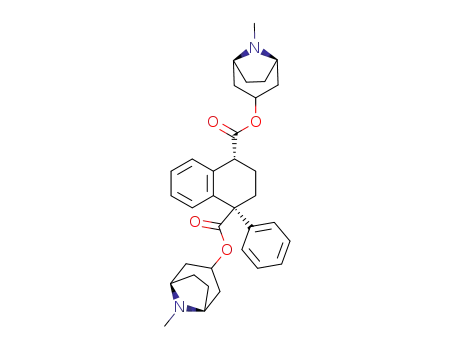
-
510-25-8,5878-33-1,6696-63-5,23852-32-6
α-Belladonnin

-
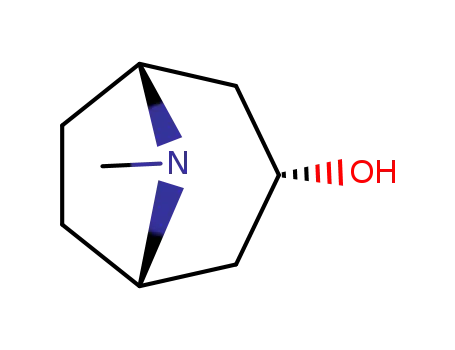
-
120-29-6
3-tropanol

-
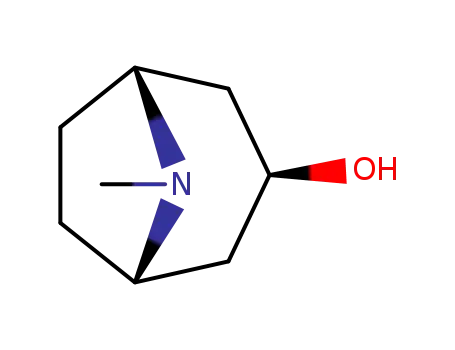
-
135-97-7
pseudotropine

-
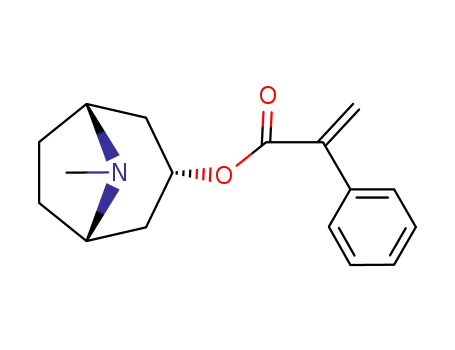
-
500-55-0
apoatropine
| Conditions | Yield |
|---|---|
|
In
water;
for 720h;
Irradiation;
decomposition in aq. solution; stability after storage in glass and polyethylene containers under various conditions (pH-value, temp., oxidative effects, irradiation);
|
532-24-1 Upstream products
-
542-05-2
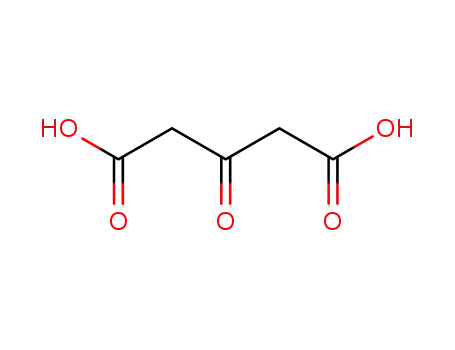
acetonedicarboxylic acid
-
638-37-9
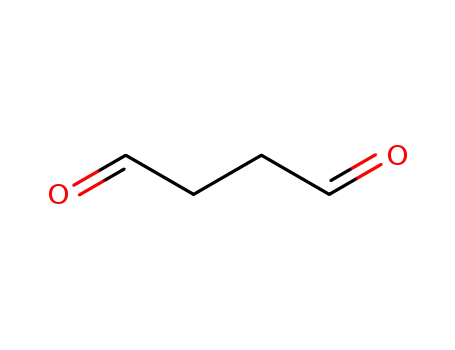
butanedial
-
593-51-1
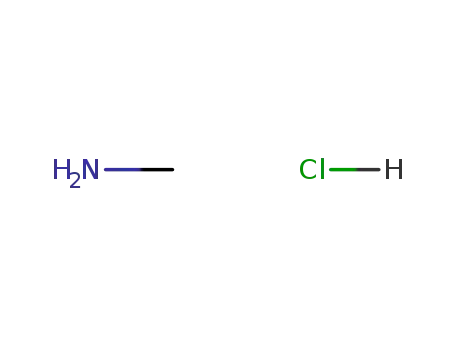
methylamine hydrochloride
-
5690-89-1
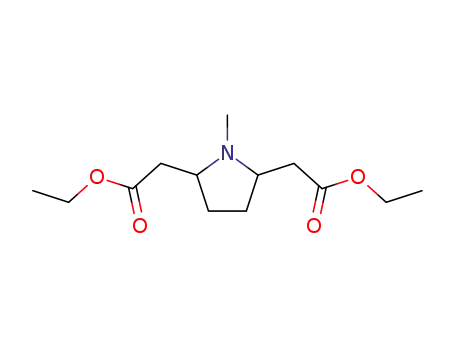
(1-methyl-pyrrolidine-2,5-diyl)-di-acetic acid diethyl ester
532-24-1 Downstream products
-
90006-89-6
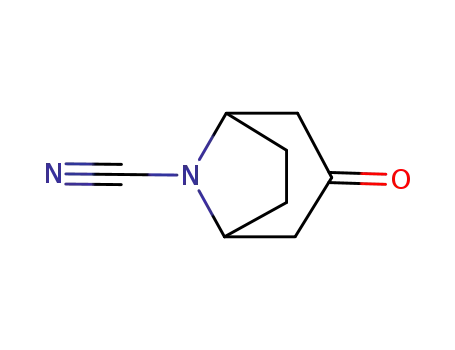
3-oxo-8-azabicyclo[3.2.1]octane-8-carbonitrile
-
109511-66-2
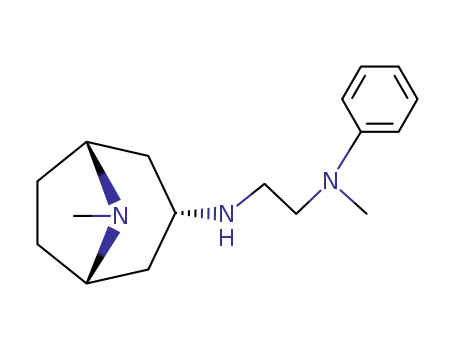
N-methyl-N-phenyl-N'-tropane-3endo-yl-ethylenediamine
-
110273-60-4
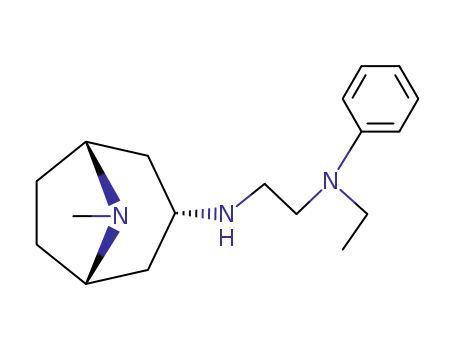
N-ethyl-N-phenyl-N'-tropane-3endo-yl-ethylenediamine
-
103153-39-5
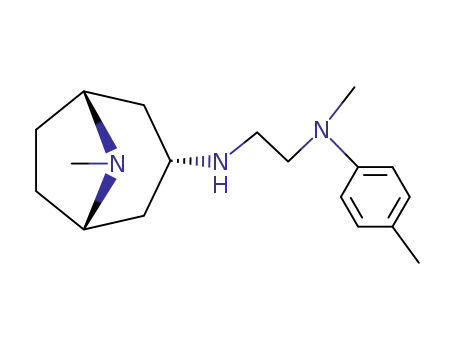
N-methyl-N-p-tolyl-N'-tropane-3endo-yl-ethylenediamine
Relevant Products
-
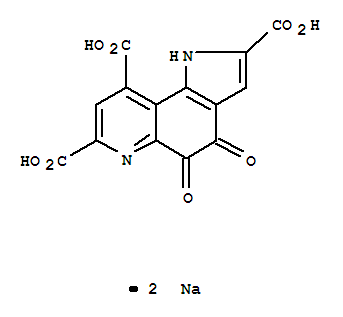
PYRROLOQUINOLINE QUINONE DISODIUM SALT
CAS:122628-50-6
-
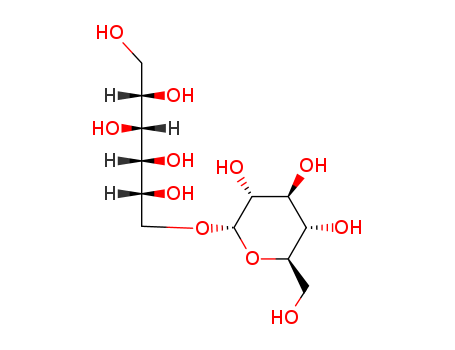
Isomaltitol
CAS:534-73-6
-
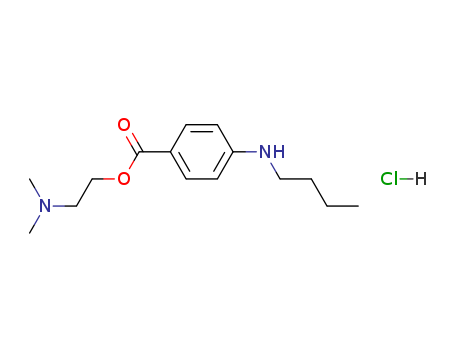
Tetracaine hydrochloride
CAS:136-47-0