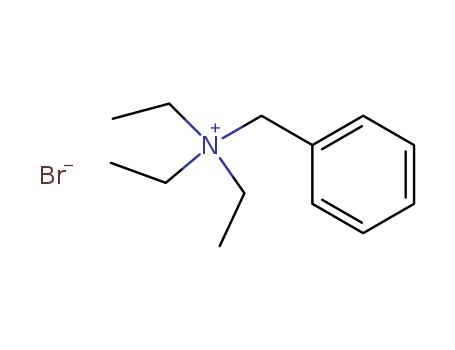
5197-95-5
- Product Name:Benzyltriethylammonium bromide
- Molecular Formula:C13H22N.Br
- Purity:99%
- Molecular Weight:272.228
Product Details;
CasNo: 5197-95-5
Molecular Formula: C13H22N.Br
Appearance: white to light yellow crystal powder
Good Supplier In China Fast Delivery Benzyltriethylammonium bromide 5197-95-5 Sales promotion qualified
- Molecular Formula:C13H22N.Br
- Molecular Weight:272.228
- Appearance/Colour:white to light yellow crystal powder
- Melting Point:193-195 °C (dec.)(lit.)
- Refractive Index:1.5260 (estimate)
- Density:1.2838 (rough estimate)
Benzyltriethylammonium bromide(Cas 5197-95-5) Usage
|
Chemical Properties |
white to light yellow crystal powde |
InChI:InChI=1/C13H22N.BrH/c1-4-14(5-2,6-3)12-13-10-8-7-9-11-13;/h7-11H,4-6,12H2,1-3H3;1H/q+1;/p-1
5197-95-5 Relevant articles
Benzylic Ammonium Ylide Mediated Epoxidations
Roiser, Lukas,Robiette, Rapha?l,Waser, Mario
supporting information, p. 1963 - 1968 (2016/08/10)
A high yielding synthesis of stilbene ox...
Gas-phase chemistry of benzyl cations in dissociation of N-Benzylammonium and N-benzyliminium ions studied by mass spectrometry
Chai, Yunfeng,Wang, Lin,Sun, Hezhi,Guo, Cheng,Pan, Yuanjiang
experimental part, p. 823 - 833 (2012/09/07)
In this study, the fragmentation reactio...
EFFECTS OF SUBSTITUENTS IN THE BENZYL BROMIDE ON THE KINETICS OF THE BENZYLATION OF AMINES
Shpan'ko, I. V.,Korostylev, A. P.,Rusu, L. N.
, p. 1715 - 1723 (2007/10/02)
The kinetics of the reactions of 3- and ...
GEMINAL SYSTEMS. 19. REACTIONS OF AMINOMETHYLPHOSPHINES WITH ELECTROPHILIC REAGENTS
Kostyanovskii, R. G.,El'natanov, Yu. I.,Shikhaliev, Sh. M.,Ignatov, S. M.,Chervin, I. I.
, p. 1433 - 1441 (2007/10/02)
-
5197-95-5 Process route
-
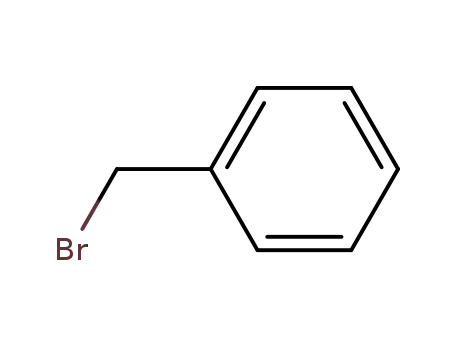
-
100-39-0
benzyl bromide

-

-
121-44-8
triethylamine

-
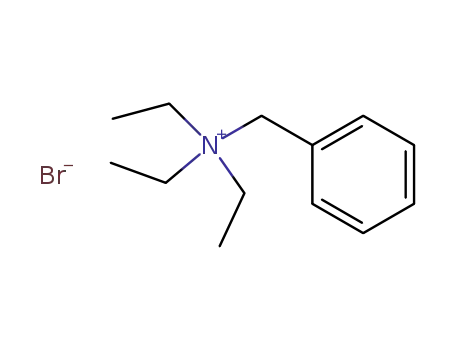
-
5197-95-5
benzyltriethylammonium bromide
| Conditions | Yield |
|---|---|
|
In
tetrahydrofuran; ethanol;
at 25 ℃;
for 24h;
Inert atmosphere;
|
98% |
|
In
1,4-dioxane; water;
at 25 ℃;
Rate constant;
α-deuterium isotope effect, further nucleophiles (SCN-, S2O3-);
|
|
|
In
nitrobenzene;
at 40 ℃;
Rate constant;
Mechanism;
|
|
|
|
|
|
at 70 ℃;
|
-
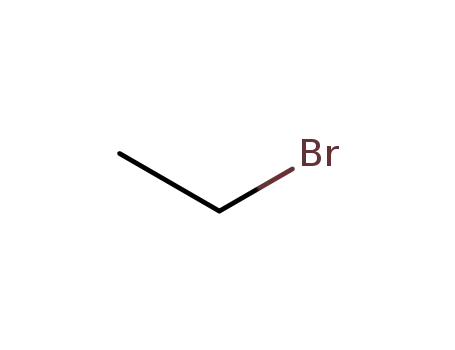
-
74-96-4
ethyl bromide

-
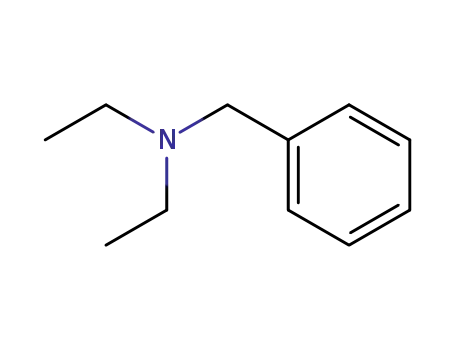
-
772-54-3
N,N-diethylbenzylamine

-
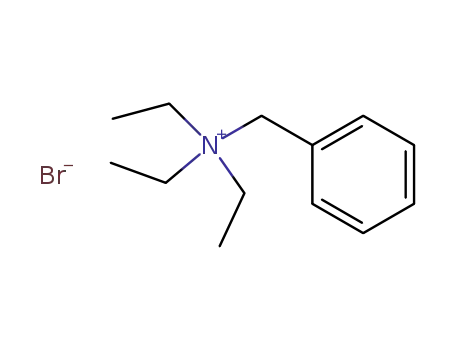
-
5197-95-5
benzyltriethylammonium bromide
| Conditions | Yield |
|---|---|
|
With
benzene;
at 100 ℃;
|
5197-95-5 Upstream products
-
74-96-4

ethyl bromide
-
772-54-3

N,N-diethylbenzylamine
-
100-39-0
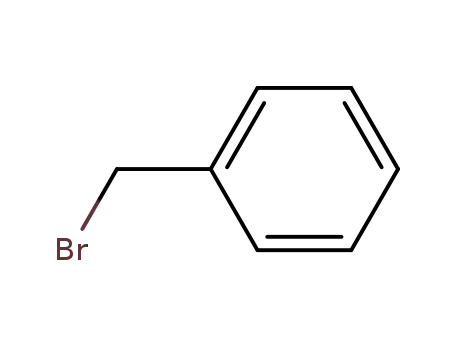
benzyl bromide
-
121-44-8

triethylamine
5197-95-5 Downstream products
-
772-54-3

N,N-diethylbenzylamine
-
103-82-2
phenylacetic acid
-
112178-91-3
5-Benzylsulfanyl-2-thioxo-[1,3]dithiole-4-carbonitrile
-
112178-88-8
(6-Amino-2-thioxo-thieno[2,3-d][1,3]dithiol-5-yl)-(4-bromo-phenyl)-methanone
Relevant Products
-
PYRROLOQUINOLINE QUINONE DISODIUM SALT
CAS:122628-50-6
-
cicloxilic acid
CAS:57808-63-6
-
Tadalafil
CAS:171596-29-5