100-09-4
- Product Name:4-methoxybenzoic acid
- Molecular Formula:C8H8O3
- Purity:99%
- Molecular Weight:152.15
Product Details;
CasNo: 100-09-4
Molecular Formula: C8H8O3
Appearance: White powder
Reliable Quality Chinese Supplier In Bulk Supply 4-methoxybenzoic acid 100-09-4
- Molecular Formula:C8H8O3
- Molecular Weight:152.15
- Appearance/Colour:White powder
- Vapor Pressure:0.002mmHg at 25°C
- Melting Point:181-186 °C
- Refractive Index:1.571-1.576
- Boiling Point:278.305 °C at 760 mmHg
- PKA:4.50(at 25℃)
- Flash Point:115.46 °C
- PSA:46.53000
- Density:1.208 g/cm3
- LogP:1.39340
- IDLH:959
- IDLH:3988
Anisic acid(Cas 100-09-4) Usage
|
Description |
4-methoxybenzoic acid, also known as p-Anisic acid or draconic acid, is an organic acid with a sweet flavor. It is one of the isomers(m-anisic acid, and o-anisic acid) of anisic acid. The term "anisic acid" often refers to this form specifically. P-anisic acid is produced through the oxidation of p-cresyl-methyl ether. |
|
Chemical Properties |
A colorless needle crystal at room temperature, soluble in ethanol, ether, chloroform, slightly soluble in hot water, insoluble in cold water. It is used as intermediate of aniracetam, and also used as fragrance and preservative. |
|
Uses |
4-Methoxybenzoic acid (p-Anisic acid) is used in oxidation and reduction of cytochrome c in solution through different self-assembled monolayers on gold electrodes using cyclic voltammetry. p-Anisic acid has antiseptic properties. It is also used as an intermediate in the preparation of more complex organic compounds. |
|
Definition |
ChEBI: 4-methoxybenzoic acid is a methoxybenzoic acid substituted with a methoxy group at position C-4. It has a role as a plant metabolite. It is functionally related to a benzoic acid. It is a conjugate acid of a 4-methoxybenzoate. |
|
Synthesis Reference(s) |
Journal of Heterocyclic Chemistry, 25, p. 973, 1988 DOI: 10.1002/jhet.5570250351Tetrahedron Letters, 34, p. 4603, 1993 DOI: 10.1016/S0040-4039(00)60635-4 |
|
General Description |
4-Methoxybenzoic acid is the sole source of carbon and energy for growth in the cultures of Nocardia sp. DSM 1069. It is effective in clearing congestion in the lungs and the respiratory tracts in conditions like asthma or bronchitis. |
|
Safety Profile |
Poison by subcutaneous route.When heated to decomposition it emits acrid smoke andirritating vapors. |
|
Who Evaluation |
Evaluation year: 2001 |
InChI:InChI=1/C8H8O3/c1-11-7-4-2-6(3-5-7)8(9)10/h2-5H,1H3,(H,9,10)
100-09-4 Relevant articles
Application of the Savage-Wood Treatment to the Quantitative Analysis of Kinetic Solvent Effects in Highly Aqueous Binary Solutions
Blokzijl, Wilfried,Jager, Jan,Engberts, Jan B. F. N.,Blandamer, Michael J.
, p. 6411 - 6413 (1986)
-
Facile electrochemical transformation of diazonium salts into carboxylic acids
Otero, M. Dolores,Batanero, Belen,Barba, Fructuoso
, p. 8215 - 8216 (2006)
The electrolyses of aryldiazonium tetraf...
Dithioester-enabled chemodivergent synthesis of acids, amides and isothiazoles via C[sbnd]C bond cleavage and C[sbnd]O/C[sbnd]N/C[sbnd]S bond formations under metal- and catalyst-free conditions
Soni, Sonam,Koley, Suvajit,Singh, Maya Shankar
, p. 2512 - 2516 (2017)
An operationally simple and user-friendl...
Hydrothermal synthesis of platinum-group-metal nanoparticles by using HEPES as a reductant and stabilizer
So, Man-Ho,Ho, Chi-Ming,Chen, Rong,Che, Chi-Ming
, p. 1322 - 1331 (2010)
Platinum-group-metal (Ru, Os, Rh, Ir, Pd...
A versatile route to the synthesis of 1-substituted β-carbolines by a single step Pictet-Spengler cyclization
Yang, Mei-Lin,Kuo, Ping-Chung,Damu, Amooru G.,Chang, Ren-Jie,Chiou, Wen-Fei,Wu, Tian-Shung
, p. 10900 - 10906 (2006)
A one-step conversion of l-tryptophan an...
Oxo osmium(VIII) complexes in oxidation: Crystal structures of OsO4·nmo (nmo = N-methylmorpholine N-oxide) and OsO4·nmm (nmm = N-methylmorpholine), and use of cis-[OsO4(OH)2]2- as an oxidation catalyst
Bailey, Alan J.,Bhowon, Minu G.,Griffith, William P.,Shoair, Abdel G. F.,White, Andrew J. P.,Williams, David J.
, p. 3245 - 3250 (1997)
The new complexes OsO4·nmo (nmo = N-meth...
A Woven Supramolecular Metal-Organic Framework Comprising a Ruthenium Bis(terpyridine) Complex and Cucurbit[8]uril: Enhanced Catalytic Activity toward Alcohol Oxidation
Li, Zhan-Ting,Liu, Yi,Wang, Hui,Wang, Ze-Kun,Xu, Zi-Yue,Zhang, Dan-Wei,Zhang, Yun-Chang
, p. 1498 - 1503 (2020)
The self-assembly of a diamondoid woven ...
Carbon nitride-catalyzed oxidative cleavage of carbon-carbon bond of α-hydroxy ketones with visible light and thermal radiation
Zhan, Haiying,Liu, Wenjie,Fu, Minling,Cen, Jinghe,Lin, Jingxin,Cao, Hua
, p. 184 - 189 (2013)
Mesoporous carbon nitride (mpg-C3N4) as ...
A General, Activator-Free Palladium-Catalyzed Synthesis of Arylacetic and Benzoic Acids from Formic Acid
Wang, Lin,Neumann, Helfried,Beller, Matthias
, p. 6910 - 6914 (2018)
A new catalyst for the carboxylative syn...
A new, highly selective synthesis of aromatic aldehydes by aerobic free-radical oxidation of benzylic alcohols, catalysed by n-hydroxyphthalimide under mild conditions. Polar and enthalpic effects
Minisci, Francesco,Punta, Carlo,Recupero, Francesco,Fontana, Francesca,Pedulli, Gian Franco
, p. 688 - 689 (2002)
A new selective synthesis of aromatic al...
Metal-Organic Framework Based on Heptanuclear Cu-O Clusters and Its Application as a Recyclable Photocatalyst for Stepwise Selective Catalysis
Zhou, Jie,Huang-Fu, Xu,Huang, Yang-Ying,Cao, Chu-Ning,Han, Jie,Zhao, Xiao-Li,Chen, Xu-Dong
, p. 254 - 263 (2020)
Visible-light driven photoreactions usin...
Palladium-catalyzed oxidative coupling of arylboronic acid with isocyanide to form aromatic carboxylic acids
Chen, Zhen-Bang,Liu, Kui,Zhang, Fang-Ling,Yuan, Qing,Zhu, Yong-Ming
, p. 8078 - 8083 (2017)
A valuable palladium-catalyzed oxidative...
Mechanochemical Grignard Reactions with Gaseous CO2 and Sodium Methyl Carbonate**
Pfennig, Victoria S.,Villella, Romina C.,Nikodemus, Julia,Bolm, Carsten
supporting information, (2022/01/22)
A one-pot, three-step protocol for the p...
A Mild Heteroatom (O -, N -, and S -) Methylation Protocol Using Trimethyl Phosphate (TMP)-Ca(OH) 2Combination
Tang, Yu,Yu, Biao
, (2022/03/27)
A mild heteroatom methylation protocol u...
Transformation of Thioacids into Carboxylic Acids via a Visible-Light-Promoted Atomic Substitution Process
Fu, Qiang,Liang, Fu-Shun,Lou, Da-Wei,Pan, Gao-Feng,Wang, Rui,Wu, Min,Xie, Kai-Jun
supporting information, p. 2020 - 2024 (2022/03/31)
A visible-light-promoted atomic substitu...
100-09-4 Process route
-
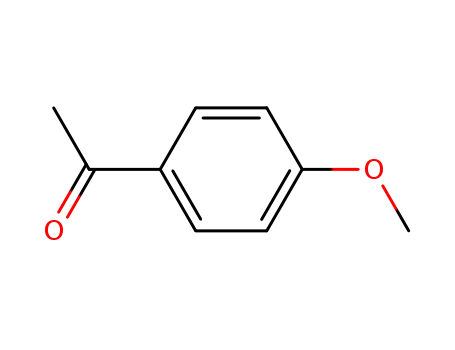
- 100-06-1
1-(4-methoxyphenyl)ethanone

-
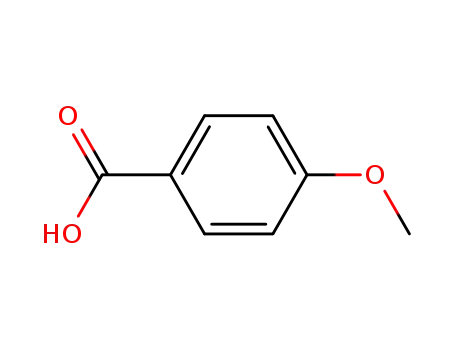
- 100-09-4
4-methoxybenzoic acid
| Conditions | Yield |
|---|---|
|
With copper(II) nitrate trihydrate; oxygen; In acetonitrile; at 120 ℃; for 10h; under 4500.45 Torr; Autoclave;
|
99% |
|
With oxygen; copper(II) nitrate; In acetonitrile; at 120 ℃; for 10h; under 4500.45 Torr;
|
99% |
|
With copper(l) iodide; hydroxylamine hydrochloride; oxygen; In dimethyl sulfoxide; at 100 ℃; for 8h; Solvent; Reagent/catalyst; Temperature;
|
95% |
|
With 1,10-Phenanthroline; oxygen; copper diacetate; potassium hydroxide; In dimethyl sulfoxide; at 130 ℃; for 12h; under 3000.3 Torr; Autoclave;
|
95% |
|
1-(4-methoxyphenyl)ethanone; With iodine; dimethyl sulfoxide; In chlorobenzene; at 130 ℃; for 3h;
With tert.-butylhydroperoxide; In chlorobenzene; at 20 - 130 ℃; for 3h; Time;
|
94% |
|
With hydroxylamine hydrochloride; iodine; In dimethyl sulfoxide; at 100 ℃; for 4h;
|
93% |
|
With sodium hypochlorite; lithium hypochlorite; In ethanol; at 77 ℃; for 2h;
|
92% |
|
With Iron(III) nitrate nonahydrate; iodine; oxygen; dimethyl sulfoxide; at 130 ℃; for 12h; under 750.075 Torr; Sealed tube; Green chemistry;
|
92% |
|
With Iron(III) nitrate nonahydrate; iodine; oxygen; In dimethyl sulfoxide; at 130 ℃; for 12h; Sealed tube;
|
92% |
|
With Oxone; trifluoroacetic acid; In 1,4-dioxane; for 10h; Reflux; Green chemistry;
|
90% |
|
With iodine; dimethyl sulfoxide; copper(II) oxide; at 90 ℃; for 3h;
|
88% |
|
With carbon tetrabromide; oxygen; In ethyl acetate; for 12h; Irradiation;
|
87% |
|
1-(4-methoxyphenyl)ethanone; With tert.-butylhydroperoxide; sodium hydroxide; tungsten(VI) oxide; In water; at 80 ℃; for 8h;
With hydrogenchloride; In water;
|
85% |
|
With oxygen; manganese (II) acetate tetrahydrate; cobalt(II) diacetate tetrahydrate; In acetic acid; at 100 ℃; for 15h; under 760.051 Torr;
|
84% |
|
With sodium percarbonate; potassium tert-butylate; sphingosylphosphorylcholine; meta-dinitrobenzene; In tert-butyl alcohol; at 80 ℃; for 5h;
|
58% |
|
With meta-dinitrobenzene; sodium hydroxide; In water; at 100 ℃; for 2.5h; Sealed tube;
|
56.2% |
|
With tert.-butylhydroperoxide; rhenium(VII) oxide; acetic acid; at 100 ℃; for 4.5h;
|
55% |
|
1-(4-methoxyphenyl)ethanone; With aluminum (III) chloride; N,N-dimethyl-aniline; In toluene; at 100 - 110 ℃; for 2 - 3h;
With hydrogenchloride; water; at 20 ℃; pH=1 - 2; Product distribution / selectivity;
|
15% |
|
With Amberlyst 15; In toluene; for 32h; Reflux;
|
10% |
|
With sodium hydroxide; chlorine;
|
|
|
With 18-crown-6 ether; acetophenone; In benzene; at 25 ℃; for 24h; Mechanism; relative reactivity; further oxidative agents;
|
|
|
With sodium hydroxide; potassium chloride; potassium hexacyanoferrate(III); In methanol; water; at 30 ℃; Kinetics; further temperature;
|
|
|
With manganese(II) nitrate; oxygen; cobalt(II) nitrate; In acetic acid; at 100 ℃; for 6h;
|
|
|
With copper(II) choride dihydrate; oxygen; lithium bromide; tert-butyl alcohol; at 130 ℃; for 10h; under 7500.75 Torr; Reagent/catalyst; Autoclave; Green chemistry;
|
|
|
With dihydrogen peroxide; In water; at 22 - 25 ℃; for 11688h;
|
|
|
Multi-step reaction with 2 steps
1: iodine; dimethyl sulfoxide / chlorobenzene / 120 °C
2: tert.-butylhydroperoxide / 6 h
With tert.-butylhydroperoxide; iodine; dimethyl sulfoxide; In chlorobenzene;
|
-
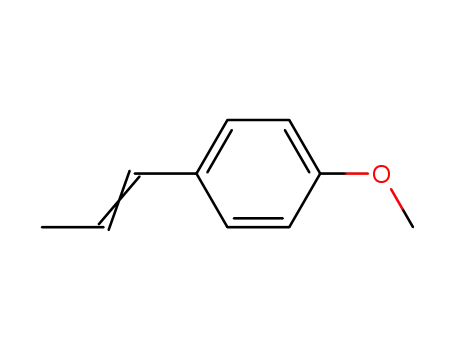
- 104-46-1,25086-72-0,63589-56-0
anethole

-
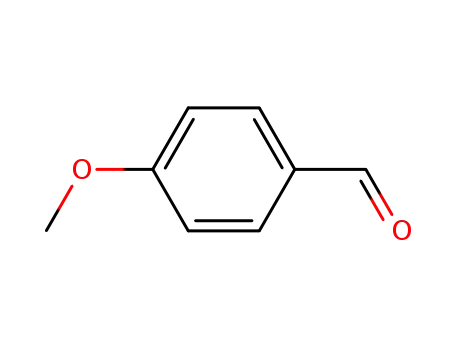
- 123-11-5
4-methoxy-benzaldehyde

-

- 100-09-4
4-methoxybenzoic acid
| Conditions | Yield |
|---|---|
|
durch elektrolytische Oxydation in neutraler, saurer oder alkalischer waessriger Suspension;
|
100-09-4 Upstream products
-
186581-53-3

diazomethane
-
2345-34-8
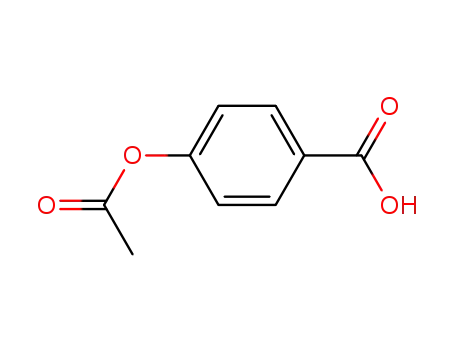
4-acetyloxy-benzoic acid
-
60-29-7
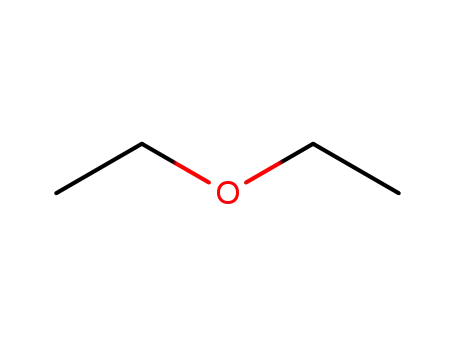
diethyl ether
-
99-96-7
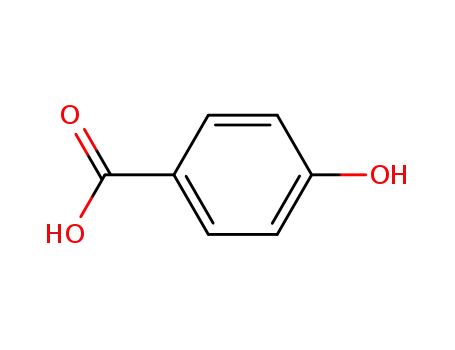
4-hydroxy-benzoic acid
100-09-4 Downstream products
-
131311-40-5
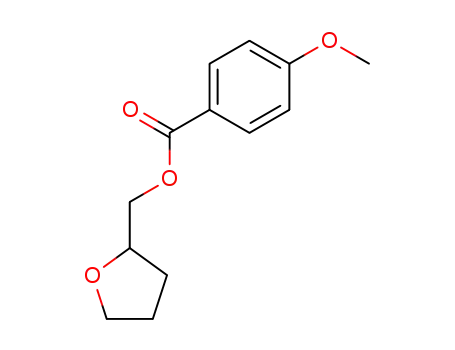
4-methoxy-benzoic acid tetrahydrofurfuryl ester
-
7465-88-5
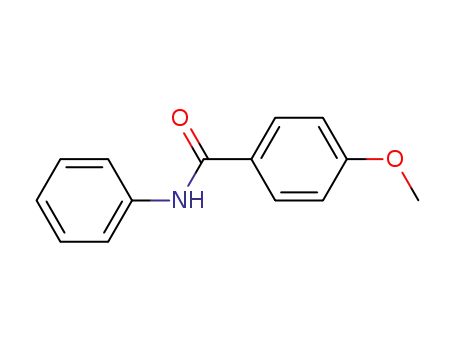
4-methoxy-N-phenylbenzamide
-
109258-04-0
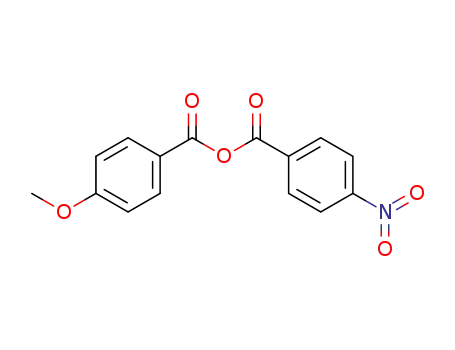
4-methoxyphenyl 4-nitrophenyl anhydride
-
37908-97-7
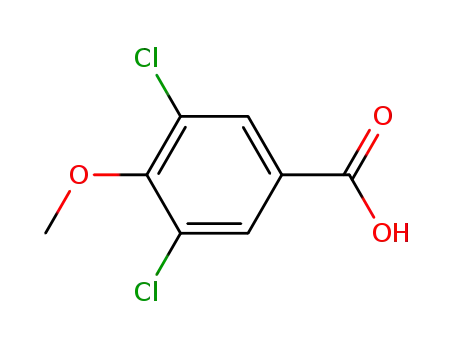
3,5-dichloro-4-methoxybenzoic acid
Relevant Products
-
Isomaltitol
CAS:534-73-6
-
alpha-Arbutin
CAS:84380-01-8
-
Bromazolam
CAS:71368-80-4