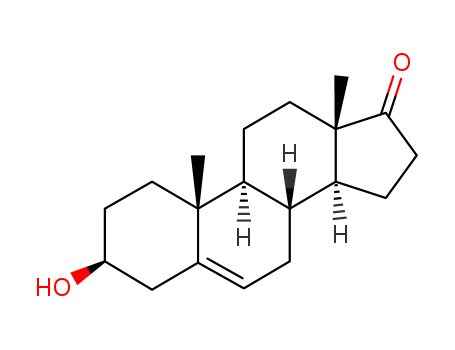
53-43-0
- Product Name:Dehydroepiandrosterone
- Molecular Formula:C19H28O2
- Purity:99%
- Molecular Weight:288.43
Product Details;
CasNo: 53-43-0
Molecular Formula: C19H28O2
Appearance: white fine crystalline powder
Hot Sale Fast Delivery Dehydroepiandrosterone 53-43-0 In Medicine
- Molecular Formula:C19H28O2
- Molecular Weight:288.43
- Appearance/Colour:white fine crystalline powder
- Vapor Pressure:4.54E-09mmHg at 25°C
- Melting Point:149-151 °C(lit.)
- Refractive Index:1.56
- Boiling Point:426.7 °C at 760 mmHg
- PKA:15.02±0.60(Predicted)
- Flash Point:182.1 °C
- PSA:37.30000
- Density:1.12 g/cm3
- LogP:3.87920
Dehydroepiandrosterone(Cas 53-43-0) Usage
|
description |
Dehydroepiandrosterone (DHEA) is a steroid hormone that is a popular nonprescription oral “dietary supplement” used by men to enhance cognitive function, mood, libido, and athletic performance. Before 1994, DHEA was a prescription medicine. After the passage of the Dietary Supplement Health and Education Act of 1994, DHEA was reclassified as a nutritional supplement. Although sold over the counter in 25- and 50-mg strengths, DHEA is widely touted to maximize results at doses of 200 mg or higher. DHEA is banned by the International Olympic Committee and the National Collegiate Athletic Association as an anabolic agent. Limited information is available regarding potential harmful effects resulting from its supplemental use. |
|
Indications and Usage |
Dehydroepiandrosterone (DHEA,) chemical name 3β-hydroxy-5alpha-androstane-17-ketone, is an esterifying 3–β–hydroxy steroid retaining 5,6 cholesterol. A white crystalline powder, soluable in ethanol, ether, and benzene, and slightly soluable in chroloform and petroleum ether. Precipitates in digitalis. DHEA is an estrogen precursor secreted by the reticular layer of the human adrenal cortex. Prevents obesity, resists diabetes, fights cancer, fights cortical disease, and delays senility treats immune deficiencies, promotes the growth and differentiation of bone cells, and promotes the synthesis of protein. It also resists viral infections, improves memory, and relieves nervous tension. DHEA is the main ingredient in steroid hormone intermediates (such as norethindrone and bisacetylene, etc.) and in birth control, and is involved in the secretion of many adrenal hormones. It has undergone extensive clinical research on treating menopausal syndrom, chorionitis, coronary heart disease, gout, psoriasis, AIDS and so on. |
|
Mechanisms of Action |
DHEA has a thyroid stimulating effect inhibiting food and fat intake and reducing fat accumulation, etc. It improves glucose tolerance, increases insulin level and fights diabetes. It can enhance endocrine system actiity, reduce cortisol levels, and resist a variety of pathological processes. It can help the body obtain cortical antibodies. DHEA has a strong protective and synergistic effect when used to treat tumors, becuase it inhibits ribose 5-phosphate. Inhibits cancer by inhibiting excessive mitochondria (NADPH) and ribose 5-phosphate esters. Regulates the growth of pancreatic cancer cells by regulating the concentration of estrogen in blood plasma. A decline in GnRH gene expression leads to aging, and DHEA can restore GnRH neuronal activity, stopping or improving certain diseases associated with declines in DHEA by stimulating GnRH biosynthesis. Restores impaired immune response, improves T- and B-cell function, and plays an important role in enhancing the physiological activity of insulin-like growth factor (IGF-1,) and is a potentially useful drug for immunodeficiency. DHEA alone cannot directly affect the growth and differential of osteoblasts, but it can do so by influencing 1,25-dihydroxyvitamin D3. The effects of DHEA on bone mass depend on the presence and form of sex hormones in bone cells and their endrocine effects on osteoblasts. DHEA is an anabolic protein hormone which promotes protein synthesis. According to the findings of Marrero and others, feeding DHEA (0.45%) to mice increased liver weight, increasing liver mitochondria by guiding liver protein restoring RNA and protein synthesis. |
|
Description |
Dehydroepiandrosterone(DHEA) is an endogenous steroid hormone that is secreted primarily by the adrenal gland and is the most abundant sex steroid. It is a neurohormone; small quantities of DHEA are produced in the brain and declines in serum and CSF with age. (Knopman and Henderson, 2003). Metabolites of DHEA include androstenedione , which subsequently may be metabolized to testosterone or estrone which is an estradiol precursor. In addition to serving as an intermediate in the biosynthesis of sex steroids, DHEA directly modulates a number of cellular and nuclear receptors. |
|
Chemical Properties |
white fine crystalline powder. There are two crystal forms. Needle-like crystal, melting point 140-141oC; small leaf-like crystal, melting point 152-153oC. Has right-handedness. Soluble in alcohol, ether, benzene, slightly soluble in chloroform, petroleum ether. In case of digitonin to produce precipitation. |
|
Originator |
Aslera ,Genelabs Technologies, Inc. |
|
Occurrence |
DHEA is naturally occurring in yam (see Wild Yam, p. 596-597). |
|
Uses |
Dehydroepiandrosterone (DHEA) is a major C19 steroid produced by the adrenal cortex. It is a major secretory steroidal product of the adrenal gland; secretion progresively declines with aging. May have estrogen-or androgen-like effects depending on the hormonal milieu. Intracellularly converted to androstenedione. It is used in treatment of menopausal syndrome. People living with HIV may be interested in taking DHEA for a variety of reasons, including treatment of depression, increasing bone density, decreasing arterial plaques, improvement of immune function with HIV, increased memory, and increased muscle strength. |
|
Definition |
ChEBI: Dehydroepiandrosterone is an androstanoid that is androst-5-ene substituted by a beta-hydroxy group at position 3 and an oxo group at position 17. It is a naturally occurring steroid hormone produced by the adrenal glands. It has a role as an androgen, a human metabolite and a mouse metabolite. It is a 17-oxo steroid, an androstanoid and a 3beta-hydroxy-Delta(5)-steroid. |
|
Manufacturing Process |
To a solution of 1 gram of 16-dehydropregnenolon-3β-acetate in 10 ml pyridine is added 0.22 gram of hydroxylamine hydrochloride, and the mixture is allowed to stand at room temperature for four days. One gram of 16dehydropregnenolon-3β-acetate oxime is dissolved in 30 ml of hot dioxane, and then the solution is cooled in an ice bath until about one-half of the dioxane has solidified. Then 1 gram of phosphorus pentachloride is added and the mixture is shaken until all the dioxane has melted. The mixture is maintained at 35°C, for seventy-five minutes, then an excess of ice is added and the solution is again allowed to stand at 35°C. After about thirty minutes, a solution of 5 ml of concentrated hydrochloric acid in 10 ml of water is added, and the mixture is diluted with water, extracted with ether and the ethereal extract washed with dilute sodium hydroxide solution. The ether is removed on a steam bath and the residue is worked up to yield dehydroepiandrosterone. |
|
Brand name |
17-chetovis 17-hormoforin;Cetavister;Climatost;Dastonil;Dha-s (prasterone);Gynodian;Longevital 5000;Maxepa;Mentalormon;Mylis;Neurocotex;Psicosterone;Ro 66827;Sh 833;Ultrapla. |
|
Therapeutic Function |
Glucocorticoid |
|
World Health Organization (WHO) |
The World Health Organization has no information further to the above regarding preparations containing prasterone or to indicate that such preparations remain available. |
|
General Description |
DHEA is a major secretory steroidal product of the adrenal gland and is a hormonal precursor to both androgens and estrogens. It can also be synthesized using wild yam or soy, but there is no evidence to show that humans are able to increase DHEA levels by consuming these foods. DHEA and its sulfated metabolite, DHEA-S, are negative modulators of GABAA receptors. |
|
Health Hazard |
An experimental teratogen.Experimental reproductive effects. Questionablecarcinogen with experimental neoplastigenic data. Whenheated to decomposition it emits acrid smoke andirritating fumes. |
|
Biochem/physiol Actions |
Product does not compete with ATP. |
|
Side effects |
Side effects may include acne, skin rash, GI upset, hirsuitism, hypertension, and increased HDL. In people with HIV, additional side effects may include fatigue, nasal congestion, and headaches. |
|
Safety |
Dehydroepiandrosterone should always be used under the supervision of a medical professional. It is likely safe for people with low DHEA levels to take oral supplements short-term (<6 months) to restore DHEA to normal, but long-term use and doses resulting in high DHEA levels are possibly unsafe. Side effects are often seen with higher doses and longterm use. |
|
references |
[1]. kimonides vg, khatibi nh, svendsen cn, et al. dehydroepiandrosterone (dhea) and dhea-sulfate (dheas) protect hippocampal neurons against excitatory amino acid-induced neurotoxicity. proc natl acad sci u s a, 1998, 95(4): 1852-1857.[2]. suzuki m, wright ls, marwah p, et al. mitotic and neurogenic effects of dehydroepiandrosterone (dhea) on human neural stem cell cultures derived from the fetal cortex. proc natl acad sci u s a, 2004, 101(9): 3202-3207.[3]. charalampopoulos i, tsatsanis c, dermitzaki e, et al. dehydroepiandrosterone and allopregnanolone protect sympathoadrenal medulla cells against apoptosis via antiapoptotic bcl-2 proteins. proc natl acad sci u s a, 2004, 101(21): 8209-8214. |
InChI:InChI=1/C19H28O2/c1-18-9-7-13(20)11-12(18)3-4-14-15-5-6-17(21)19(15,2)10-8-16(14)18/h3,13-16,20H,4-11H2,1-2H3/t13-,14-,15?,16?,18-,19-/m0/s1
53-43-0 Relevant articles
Synthesis and structure determination of 3β-hydroxyandrost-5-en-17-one (C19H28O2·CH3OH)
Verma, Rajnikant,Jasrotia, Dinesh,Sawhney, Anshu,Bhat, Mousmi,Gupta
, p. 523 - 528 (2004)
3β-Hydroxyandrost-5-en-17-one (C19H28O 2...
A steroidogenic pathway for sulfonated steroids: The metabolism of pregnenolone sulfate
Neunzig,Sánchez-Guijo,Mosa,Hartmann,Geyer,Wudy,Bernhardt
, p. 324 - 333 (2014)
In many tissues sulfonated steroids exce...
Isolation of new androstenol-3()-on-17 from the urine of a patient with
DINGEMANSE,HUIS IN T VELD,HARTOGH-KATZ
, p. 848 - 848 (1948)
-
Etherification of Hydroxystereoids via Triflates
Belostotskii, Anatoly M.,Hassner, Alfred
, p. 5075 - 5076 (1994)
Triflates of saturated alcohols are usef...
Modified bile acids and androstanes—Novel promising inhibitors of human cytochrome P450 17A1
Dzichenka, Yaraslau,Shapira, Michail,Yantsevich, Aliaksei,Cherkesova, Tatsiana,Grbovi?, Ljubica,Savi?, Marina,Usanov, Sergey,Jovanovi?-?anta, Suzana
, (2020/11/17)
Cytochromes P450 are key enzymes for ste...
Deconstructive Oxygenation of Unstrained Cycloalkanamines
Han, Bing,He, Yi-Heng,Pan, Jia-Hao,Wang, Yuan-Rui,Yu, Wei,Zhang, Jian-Wu
, p. 3900 - 3904 (2020/02/11)
A deconstructive oxygenation of unstrain...
Comprehensive kinetic and substrate specificity analysis of an arylsulfatase from Helix pomatia using mass spectrometry
Correia, Mário S.P.,Ballet, Caroline,Meistermann, Hannes,Conway, Louis P.,Globisch, Daniel
, p. 955 - 962 (2019/02/09)
Sulfatases hydrolyze sulfated metabolite...
Method for preparing 4-androstenedione from dehydroepiandrosterone acetate
-
Paragraph 0026; 0027; 0028; 0032-0034; 0038-0040, (2019/07/04)
The invention provides a method for prep...
53-43-0 Process route

-
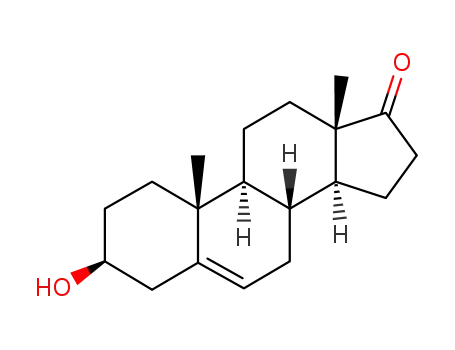
-
53-43-0
dehydroepiandrosterone

-
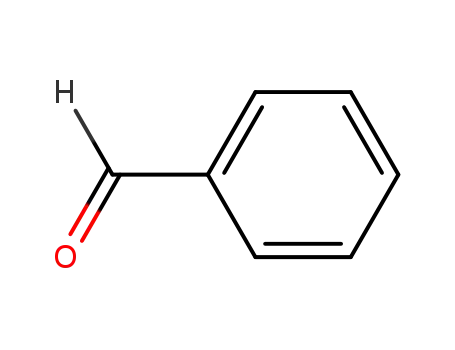
-
100-52-7
benzaldehyde
| Conditions | Yield |
|---|---|
|
|
-
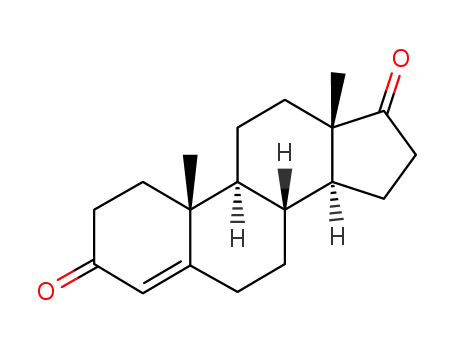
-
63-05-8
Androstenedione

-

-
53-43-0
dehydroepiandrosterone
| Conditions | Yield |
|---|---|
|
Androstenedione;
With
titanium(IV) oxide; platinum;
In
toluene;
at 100 ℃;
for 3h;
With
hydrogen;
In
toluene;
for 8h;
under 15001.5 Torr;
Temperature;
Reagent/catalyst;
Pressure;
|
85% |
|
Multi-step reaction with 2 steps
1.1: potassium tert-butylate / tert-butyl alcohol / 1.5 h / 35 - 40 °C / Inert atmosphere
1.2: 20 - 25 °C
2.1: NADP; magnesium(II) chloride hexahydrate; alpha-D-glucopyranose; NAD+ / 2-methyltetrahydrofuran; aq. phosphate buffer / 20 - 32 °C / Enzymatic reaction
With
alpha-D-glucopyranose; magnesium(II) chloride hexahydrate; potassium tert-butylate; NADP; NAD+;
In
2-methyltetrahydrofuran; aq. phosphate buffer; tert-butyl alcohol;
|
|
|
Multi-step reaction with 2 steps
1: potassium tert-butylate / tert-butyl alcohol / 1 h / 30 - 35 °C / Inert atmosphere
2: D-glucose; NAD; glucose dehydrogenase (CDX-901)_; ketoreductase from Sphingomonas wittichii; potassium carbonate / ethyl acetate; aq. phosphate buffer / 21 h / 32.5 °C / pH 6.3 - 6.5 / Inert atmosphere; Enzymatic reaction
With
D-glucose; glucose dehydrogenase (CDX-901)_; ketoreductase from Sphingomonas wittichii; potassium tert-butylate; NAD; potassium carbonate;
In
aq. phosphate buffer; ethyl acetate; tert-butyl alcohol;
|
|
|
Multi-step reaction with 4 steps
1: aluminum (III) chloride; 5-sulfosalicylic Acid / 5 h / 20 - 30 °C / Inert atmosphere; Large scale
2: orthoformic acid triethyl ester; boron trifluoride diethyl etherate / dichloromethane / 5 h / 20 - 30 °C / Inert atmosphere
3: calcium borohydride / dichloromethane / 15 h / -20 - -15 °C
4: toluene-4-sulfonic acid / acetone; water / 1 h / 45 - 50 °C / Inert atmosphere
With
aluminum (III) chloride; calcium borohydride; boron trifluoride diethyl etherate; toluene-4-sulfonic acid; orthoformic acid triethyl ester; 5-sulfosalicylic Acid;
In
dichloromethane; water; acetone;
|
53-43-0 Upstream products
-
57-88-5
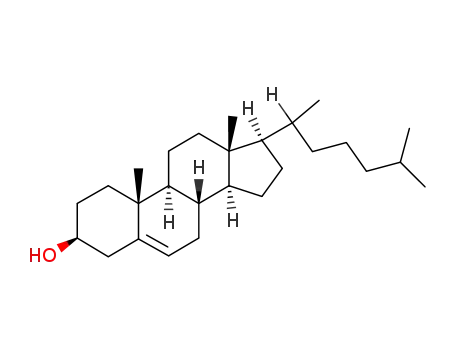
cholesterol
-
83-46-5
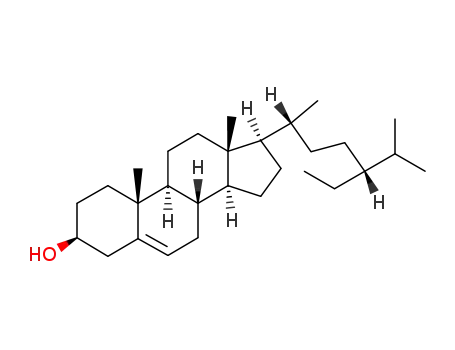
β-sitosterol
-
651-48-9
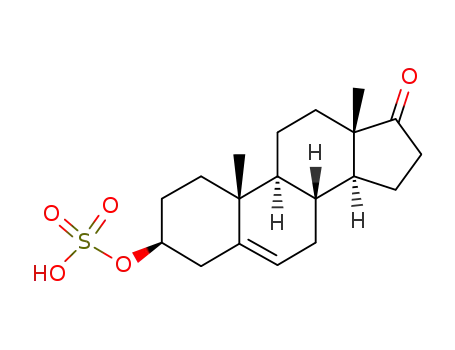
dehydroepiandrosterone sulfate
-
571-36-8
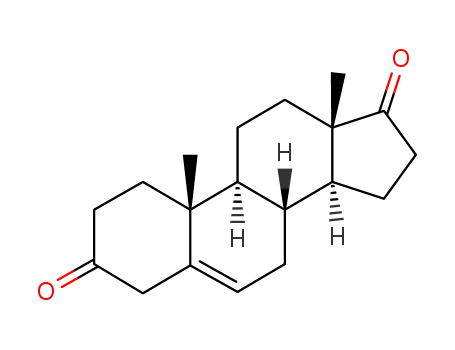
5-androstenedione
53-43-0 Downstream products
-
5419-51-2
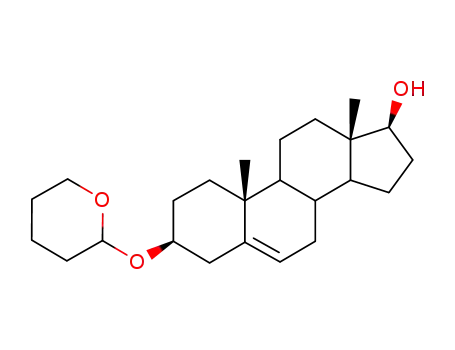
3β-(2-tetrahydropyranyloxy)-5-androstene-17β-0l
-
566-19-8
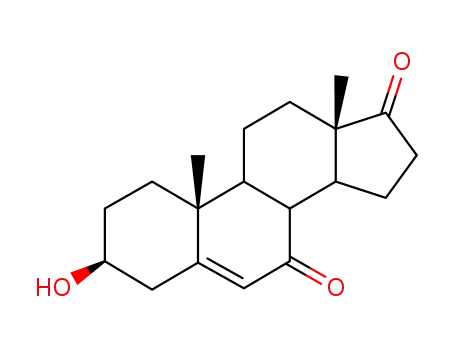
3β-hydroxyandrost-5-ene-7,17-dione
-
628-13-7
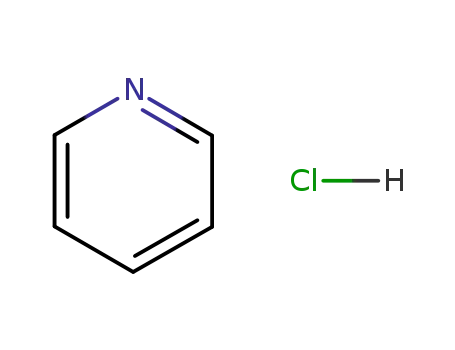
pyridine hydrochloride
-
521-17-5
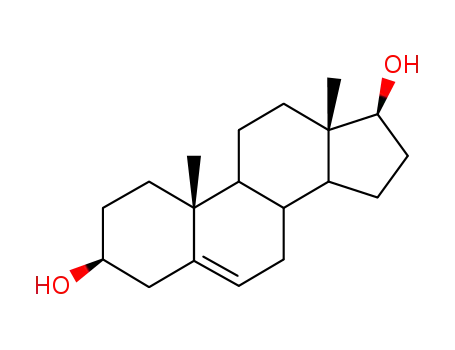
androst-5-ene-3β,17β-diol
Relevant Products
-
Isomaltitol
CAS:534-73-6
-
CJC-1295
CAS:863288-34-0
-
Somatotropin
CAS:12629-01-5