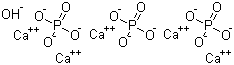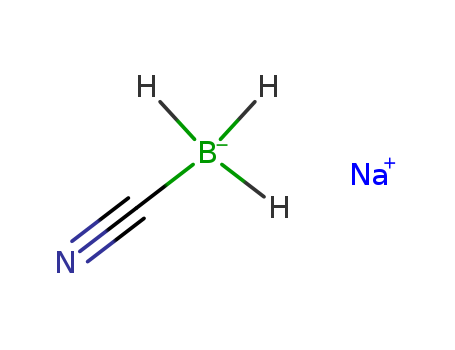
25895-60-7
- Product Name:Sodium cyanoborohydride
- Molecular Formula:NaBH3CN
- Purity:99%
- Molecular Weight:62.8423
Product Details;
CasNo: 25895-60-7
Molecular Formula: NaBH3CN
Appearance: white crystalline powder
High Purity Sodium cyanoborohydride 25895-60-7 In Bulk Supply with Wholesale Price
- Molecular Formula:NaBH3CN
- Molecular Weight:62.8423
- Appearance/Colour:white crystalline powder
- Melting Point:>242°C (dec.)(lit.)
- Boiling Point:307°C
- Flash Point:?1°F
- PSA:23.79000
- Density:1.083 g/mL at 25 °C
- LogP:-1.16712
Sodium cyanoborohydride(Cas 25895-60-7) Usage
|
Description |
Sodium cyanoborohydride is valued in organic synthesis due to its ability to selectively reduce carbonyl compounds and its compatibility with a range of reaction conditions. Sodium cyanoborohydride can be synthesized by stirring equimolar BH3·THF (~1 M) with NaCN in THF (tetrahydrofuran) in excellent yield. The synthesis typically involves a reaction between sodium borohydride and a solution of hydrogen cyanide in THF. |
|
Uses |
Sodium cyanoborohydride can replace its hydrogen atoms with deuterium (D) or tritium (T) through reactions with deuterated water (D2O) or tritiated water. Sodium cyanoborohydride is used in the synthesis of chemical models, such as phenolate-bridged dilanthanum(III) complexes. These complexes are of interest as models for metalloproteins, illustrating the compound's importance in both basic and applied chemistry. Sodium cyanoborohydride is commonly used in reductive amination reactions. This process is essential in organic synthesis for introducing amine groups into molecules, allowing for the conversion of carbonyl compounds into their corresponding amines. It is particularly effective for reducing aldehydes and ketones. |
InChI:InChI=1/CH3BN.Na/c2-1-3;/h2H3;/q-1;+1
25895-60-7 Relevant articles
Sodium Cyanoborohydride - A Highly Selective Reducing Agent for Organic Functional Groups
Clinton F. LANE*
, Synthesis 1975; 1975(3): 135-146
Firstly a summary of the preparation and properties of sodium cyanoborohydride is given. Then some examples of sodium cyanoborohydride reductions of various systems are given including some applications of sodium cyanoborodeuteride.
Studies of the mechanistic diversity of sodium cyanoborohydride reduction of tosylhydrazones
Vaughn P. Miller, Ding Yah Yang, Theresa M. Weigel, Oksoo Han, and Hung Wen Liu
, J. Org. Chem. 1989, 54, 17, 4175–4188
Reduction of tosylhydrazone derivatives of ketones and aldehydes with sodium cyanoborohydride in acidic medium is a mild, albeit versatile, deoxygenationreaction.
An improved method for reductive alkylation of amines using titanium(IV) isopropoxide and sodium cyanoborohydride
Ronald J. Mattson, Kahnie M. Pham, David J. Leuck, and Kenneth A. Cowen
, J. Org. Chem. 1990, 55, 8, 2552–2554
Sodium cyanoborohydride via the general procedure to give … to generate enamines which were then reduced by the sodium cyanoborohydride. We were …
25895-60-7 Upstream products
-
846060-69-3
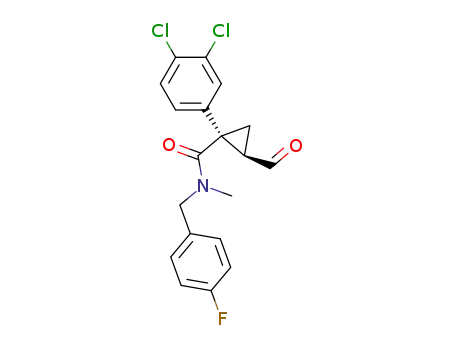
(1S,2R)-1-(3,4-dichlorophenyl)-2-formyl-cyclopropanecarboxylic acid (4-fluorobenzyl)methylamide
-
52509-14-5
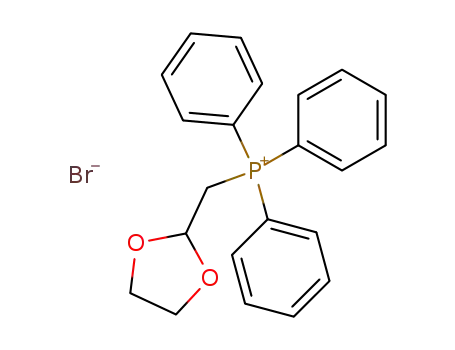
(1,3-dioxolan-2-yl-methyl)triphenylphosphonium bromide
-
16940-66-2
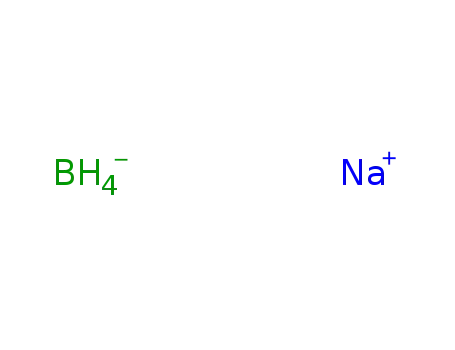
sodium tetrahydroborate
-
74-90-8

hydrogen cyanide
25895-60-7 Downstream products
-
82176-64-5
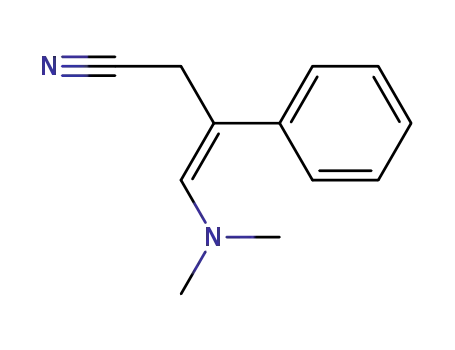
(Z)-4-Dimethylamino-3-phenyl-but-3-enenitrile
-
82176-65-6
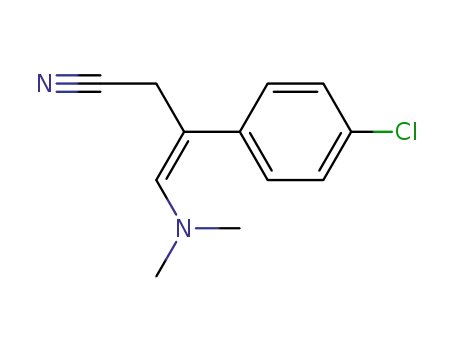
(Z)-3-(4-Chloro-phenyl)-4-dimethylamino-but-3-enenitrile
-
82176-66-7
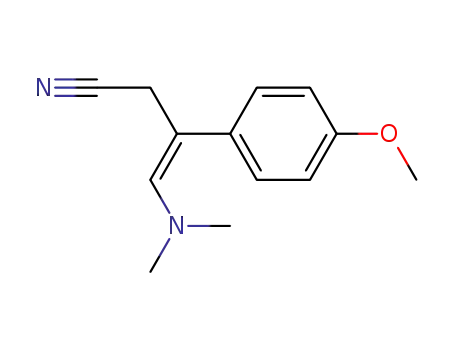
(Z)-4-Dimethylamino-3-(4-methoxy-phenyl)-but-3-enenitrile
-
70700-36-6
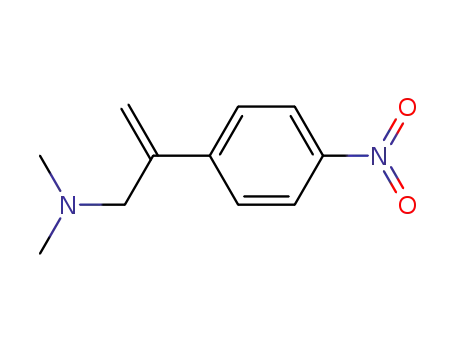
N,N-Dimethyl-β-methylen-4-nitro-phenylethane amine
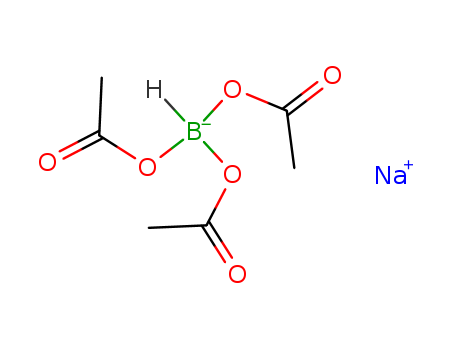
Relevant Products
-
Sodium triacetoxyborohydride
CAS:56553-60-7
-
Hydroxyapatite
CAS:1306-06-5
-
Methyl pyruvate
CAS:600-22-6