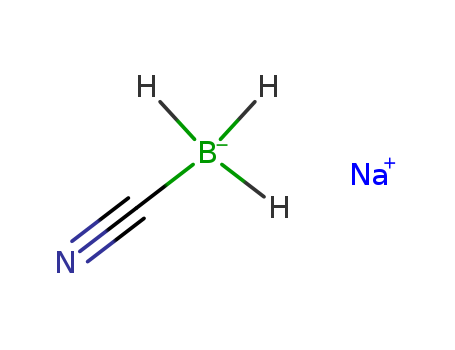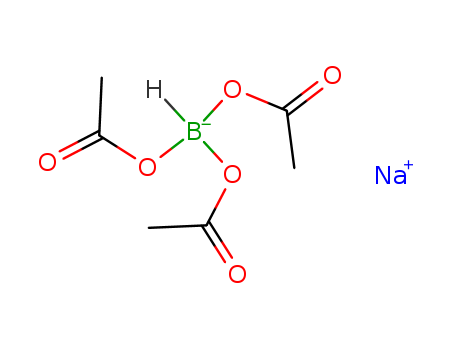
56553-60-7
- Product Name:Sodium triacetoxyborohydride
- Molecular Formula:C6H10BNaO6
- Purity:99%
- Molecular Weight:211.943
Product Details;
CasNo: 56553-60-7
Molecular Formula: C6H10BNaO6
Appearance: white crystalline powder
Best Price Good Producer Sodium triacetoxyborohydride 56553-60-7 In Medicine
- Molecular Formula:C6H10BNaO6
- Molecular Weight:211.943
- Appearance/Colour:white crystalline powder
- Vapor Pressure:0Pa at 25℃
- Melting Point:116-120 °C (dec.)(lit.)
- Boiling Point:111.1℃[at 101 325 Pa]
- PSA:78.90000
- Density:1.36[at 20℃]
- LogP:-0.60710
Sodium triacetoxyborohydride(Cas 56553-60-7) Usage
|
Chemical Properties |
Sodium triacetoxyborohydride (STAB-H) is commercially available as a hygroscopic white powder with a melting point of 116-120 °C. freely soluble in benzene. It is prepared by the reaction of NaBH4 with excess acetic acid in benzene or toluene. |
|
Uses |
Sodium Triacetoxyborohydride(STAB) is a hydride reagent used in stereoselective reductive amination. It is able to replace toxic sodium cyanoborohydride under most conditions. It is selective in reducing aldehydes to alcohols in the presence of ketones. STAB is also stable in anhydrous acids, which enables reductive amination of aldehydes and ketones. It used in reductive amination of ketones and aldehydes and reductive amination/lactamization of carbonyl compounds with amines. The advantage of STAB compared to sodium cyanoborohydride is evident. STAB, being non-toxic, is easier to handle and forms no toxic by-products, making the treatment of process waste after the reaction simple and less costly. |
|
Application |
Sodium triacetoxyborohydride is a mild reagent that exhibits remarkable selectivity as a reducing agent. It reduces aldehydes but not ketones; however, beta-hydroxyketones can be reduced selectively to give 1,3-trans diols.The steric and the electron-withdrawing effects of the three acetoxy groups stabilize the boron-hydrogen bond and are responsible for its mild reducing properties.Sodium triacetoxyborohydride or [NaBH(OAc)3] can be used as a reagent:In the reductive amination of ketones and aldehydes.To prepare N-benzyl-γ-valerolactam by reacting with methyl 4-oxopentanoate and benzylamine via reductive amination/lactamization.To reduce imines and enamines to corresponding amines.To reduce quinolines and isoquinolines to corresponding tetrahydro derivatives.In the hydroboration of alkenes.To synthesize nitroxide biradicals for creating high relaxivity terminal groups linkage to dendrimers. |
|
Reactions |
Sodium Triacetoxyborohydride is selective reducing agent in organic synthesis. It is especially suitable for reductive aminations. Since the reaction rate for the reduction of iminium ions is much faster than for ketones or even aldehydes, the reductive amination can be carried out as a one-pot procedure by introducing the reducing agent into a mixture of the amine and carbonyl compound. The presence of a stoichiometric amount of acetic acid, which catalyzes the imine formation and provides the iminium ion, doesn't present any problem under these conditions.Reductive amination (simplified) |
InChI:InChI=1/C6H10BO6.Na/c1-11-4(8)7(5(9)12-2)6(10)13-3;/h7H,1-3H3;/q-1;+1/rC6H10BNaO6/c1-12-4(9)7(8,5(10)13-2)6(11)14-3/h7H,1-3H3
56553-60-7 Relevant articles
A β-enaminone-initiated multicomponent domino reaction for the synthesis of indoloquinolizines and benzoquinolizines from acyclic precursors
Suryavanshi, Padmakar A.,Sridharan, Vellaisamy,Menéndez, J. Carlos
, p. 13207 - 13215 (2013)
The cerium(IV) ammonium nitrate (CAN)-ca...
Synthesis from (+)-α-pinene of optically active macrocycles containing cyclobutane, ester, azine, or hydrazide groups
Ishmuratov,Mingaleeva,Shakhanova,Muslukhov,Yakovleva,Botsman,Tolstikov
, p. 210 - 214 (2011)
Optically active symmetric macrocyclic d...
Application of thermal analytical techniques in development of a safe and robust process for production of triacetoxyborohydride (STAB)
Lam, Thientu T.,Bagner, Carl,Tuma, Linda
, p. 109 - 113 (2005)
The thermal hazards associated with the ...
Two-step stereocontrolled synthesis of densely functionalized cyclic β-aminoesters containing four stereocenters, based on a new cerium(IV) ammonium nitrate catalyzed sequential three-component reaction
Sridharan, Vellaisamy,Menendez, J. Carlos
, p. 4303 - 4306 (2008)
(Chemical Equation Presented) The cerium...
Total Synthesis of Ritterazine B
Nakayama, Yasuaki,Maser, Michael R.,Okita, Tatsuya,Dubrovskiy, Anton V.,Campbell, Taryn L.,Reisman, Sarah E.
supporting information, p. 4187 - 4192 (2021/04/06)
The first total synthesis of the cytotox...
Structure-Activity Relationship Studies of Pyrimidine-4-Carboxamides as Inhibitors of N-Acylphosphatidylethanolamine Phospholipase D
Mock, Elliot D.,Kotsogianni, Ioli,Driever, Wouter P. F.,Fonseca, Carmen S.,Vooijs, Jelle M.,Den Dulk, Hans,Van Boeckel, Constant A. A.,Van Der Stelt, Mario
, p. 481 - 515 (2021/02/05)
N-Acylphosphatidylethanolamine phospholi...
Synthesis of 1,4-Diazepanes and Benzo[ b][1,4]diazepines by a Domino Process Involving the in Situ Generation of an Aza-Nazarov Reagent
Maiti, Swarupananda,Leonardi, Marco,Cores, ángel,Tenti, Giammarco,Ramos, M. Teresa,Villacampa, Mercedes,Menéndez, J. Carlos
, p. 11924 - 11933 (2020/10/23)
A step-and atom-economical protocol allo...
Low-cost method preparation for doxazosin mesylate controlled release tablets
-
Paragraph 0017; 0018, (2019/07/01)
The invention relates to a preparation m...
56553-60-7 Process route
-
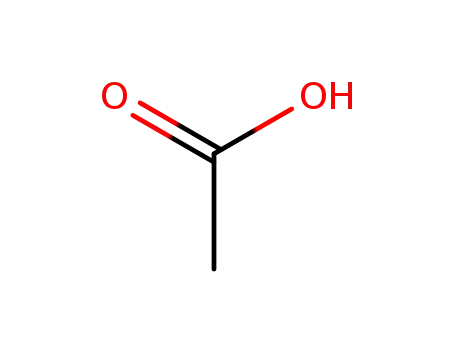
-
64-19-7,77671-22-8
acetic acid

-
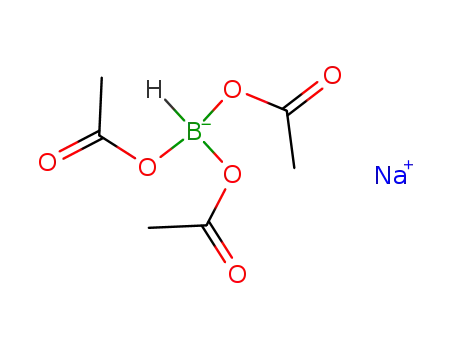
-
56553-60-7
sodium tris(acetoxy)borohydride
| Conditions | Yield |
|---|---|
|
With
sodium tetrahydroborate;
In
benzene;
|
|
|
With
sodium tetrahydroborate;
at 15 - 20 ℃;
|
|
|
With
sodium tetrahydroborate;
In
2-methylpropyl acetate;
at 0 - 5 ℃;
for 1h;
|
|
|
With
sodium tetrahydroborate;
In
dichloromethane;
for 2h;
|
|
|
With
sodium tetrahydroborate;
In
tetrahydrofuran;
at 0 - 20 ℃;
Inert atmosphere;
Schlenk technique;
|
-
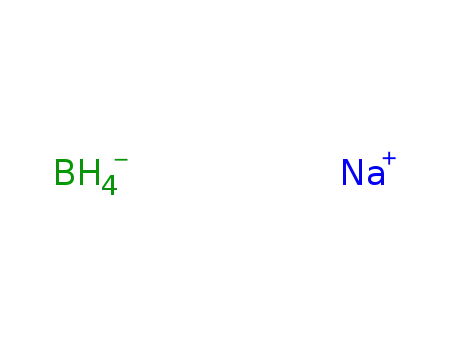
-
16940-66-2
sodium tetrahydroborate

-

-
56553-60-7
sodium tris(acetoxy)borohydride
| Conditions | Yield |
|---|---|
|
With
acetic acid;
In
benzene;
byproducts: H2; under N2-atmosphere; slurry of NaBH4 in benzene cooled (10°C); CH3COOH added dropwise (temp. < 20°C); mixt. warmed (ambient temp.) and stirred for 8h;; filtration; white powder washed (ether) and held under vacuum over night; elem. anal.;;
|
92% |
56553-60-7 Upstream products
-
64-19-7

acetic acid
-
4887-24-5
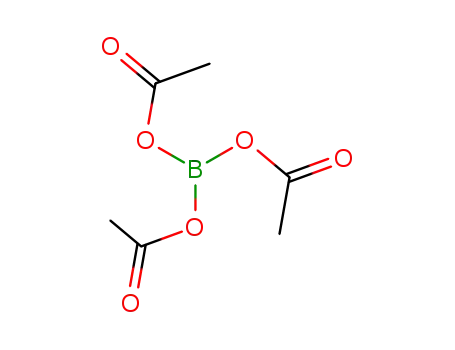
triacetoxyborane
-
7646-69-7
sodium hydride
-
16940-66-2
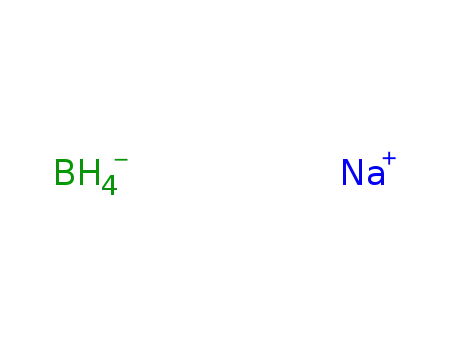
sodium tetrahydroborate
56553-60-7 Downstream products
-
447454-16-2
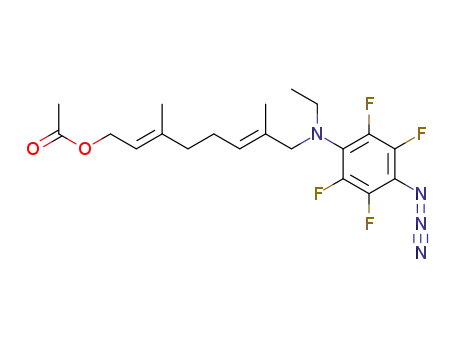
8-(p-azido-N-ethyl-tetrafluoroaniline)-3,7-dimethyl-1-acetoxyl-2,6-octadiene
-
717105-53-8
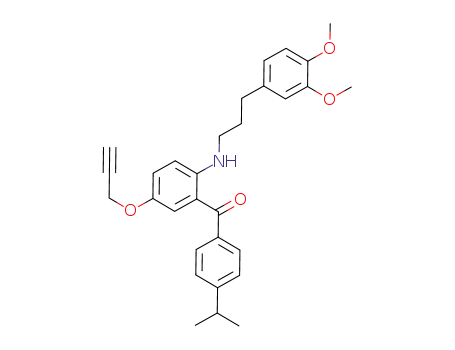
{2-[3-(3,4-dimethoxy-phenyl)-propylamino]-5-propargyloxy-phenyl}-(4-isopropyl-phenyl)-methanone
-
143878-86-8
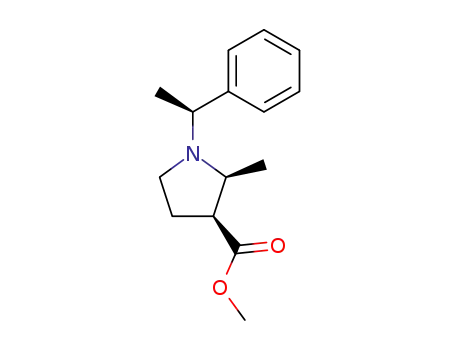
(2S,3S)-<1-(1(S)-phenylethyl)-2-methylpyrrolidin-3-yl>carboxylic acid methyl ester
Relevant Products
-
Sodium cyanoborohydride
CAS:25895-60-7
-
Ammonium dihydrogen phosphate
CAS:7722-76-1
-
Ethoxyquin
CAS:91-53-2