105-41-9
- Product Name:1,3-dimethylpentylamine
- Molecular Formula:C7H17N
- Purity:99%
- Molecular Weight:115.219
Product Details;
CasNo: 105-41-9
Molecular Formula: C7H17N
Appearance: white or category of white crystal powder
High Quality Chinese Supplier Export 1,3-dimethylpentylamine 105-41-9
- Molecular Formula:C7H17N
- Molecular Weight:115.219
- Appearance/Colour:white or category of white crystal powder
- Vapor Pressure:0.651mmHg at 25°C
- Melting Point:120-130 °C
- Refractive Index:n20/D1.417-1.419
- Boiling Point:133.9 °C at 760 mmHg
- PKA:pKa 10.54(H2O,t =24±1,I=0.002) (Uncertain)
- Flash Point:28.9 °C
- PSA:26.02000
- Density:0.775 g/cm3
- LogP:2.47010
1,3-Dimethylpentylamine(Cas 105-41-9) Usage
|
Description |
1,3-dimethylamylamine or methylexaneamine (DMAA) is a synthetic pharmaceutical patented in the 1940s as a nasal decongestant which can be used as a recreational stimulant. Alleged to occur in nature,DMAA has become a widely used ingredient in sports food supplements, despite its status as a doping agent and concerns over its safety. The most popular brands containing DMAA include Jack3d? and OxyElite Pro?, both marketed by USP Labs and both detained by the FDA in July 2013 (however, some can still be found on Amazon and other online retail stores). |
|
Chemical Properties |
White or almost white powder |
|
Originator |
Forthane,Lilly,US,1948 |
|
Uses |
DMAA (1,3-dimethylamylamine) is an amphetamine derivative that has been widely used in sports supplements sold in the United States. Also known as methylhexanamine or geranium extract, DMAA is often touted as a "natural" stimulant, with many claimed functional uses including a body-building aid, an athletic performance enhancer, and a weight-loss aid. Although DMAA at one time was approved as a drug for nasal decongestion, no medical use of DMAA is recognized today. FDA is not aware of any reliable science indicating that DMAA exists naturally in plants. |
|
Application |
1,3-Dimethylamylamine (1,3-DMAA) is a neural stimulant with a structure similar to ephedrine and adrenaline that has been used as a pre-workout stimulant. It is useful in compositions, for example as dietary supplements, and for appetite suppression. Methylhexaneamine is a botanical ingredient in dietary supplements. Methylhexaneamine and caffeine has been combined in attempts to improve exercise performance and related variables. |
|
Manufacturing Process |
One molecular equivalent of 4-methylhexanone-2 is reacted with slightly more than one molecular equivalent of hydroxylamine. Desirably, the hydroxylamine is prepared in the presence of the 4-methylhexanone-2 by reacting the hydrochloride or sulfate or other salt of the hydroxylamine with a suitable base, such as sodium carbonate or sodium hydroxide. Desirably, the reaction mixture is agitated for a few hours to insure the conversion of the 4- methylhexanone-2 to 4-methylhexanone-2 oxime.The resulting 4-methylhexanone-2 oxime separates and is dried by any suitable means, such as with a dehydrating agent, for example, sodium sulfate or magnesium sulfate. After drying, 4-methylhexanone-2 oxime is reduced with hydrogen by means of a catalyst, such as Raney nickel, or by reaction of sodium and a primary alcohol, such as ethanol. The resulting 2- amino-4-methylhexane may be purified by distillation, as described in US Patent 2,350,318.115 g (1 mol) of 2-amino-4-methylhexane and 9 g (0.5 mol) of water are placed in a tared 500 cc 3-necked flask which is equipped with a mechanical stirrer, a thermometer, and a gas delivery tube. The flask is surrounded by a cooling bath of ice and water. Dry carbon dioxide gas is introduced into the solution through the gas delivery tube, with constant stirring, until the increase in weight is approximately 22 g (0.5 mol). The temperature during this addition is maintained between 20° and 30°C. A viscous liquid results, and consists essentially of 2-amino-4-methylhexane carbonate. This also dissociates very slowly at room temperature to the free amine, carbon dioxide, and water; and is effective as an inhalant, according to US Patent 2,386,273. |
|
Therapeutic Function |
Nasal decongestant |
InChI:InChI=1/C7H17N.ClH/c1-4-6(2)5-7(3)8;/h6-7H,4-5,8H2,1-3H3;1H
105-41-9 Process route
-
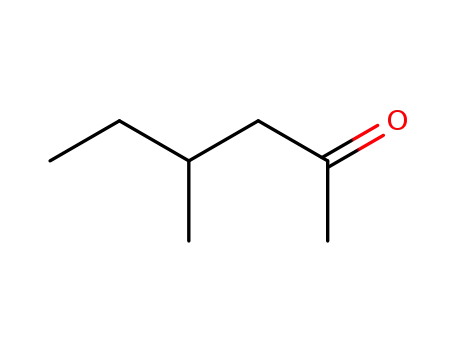
- 105-42-0
4-methylhexan-2-one

-
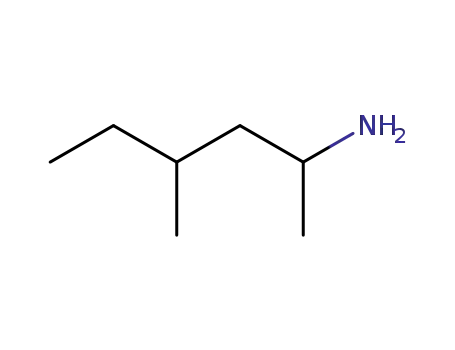
- 105-41-9
1,3-dimethylamylamine
| Conditions | Yield |
|---|---|
|
|

-

- 105-41-9
1,3-dimethylamylamine
| Conditions | Yield |
|---|---|
|
With ethanol; sodium; toluene;
|
105-41-9 Upstream products
-
105-42-0

4-methylhexan-2-one
Relevant Products
-
Tropinone
CAS:532-24-1
-
Stanozolol
CAS:10418-03-8
-
α-bromohexanophenone
CAS:59774-06-0