366-18-7
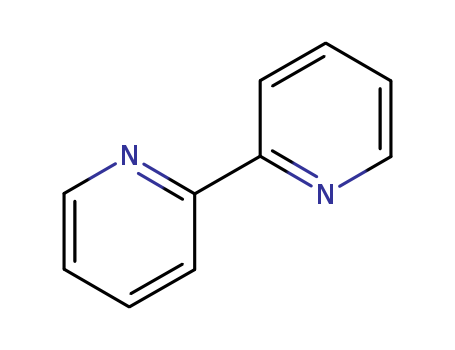
- Product Name:2,2'-Bipyridine
- Molecular Formula:C10H8N2
- Purity:99%
- Molecular Weight:157.195
Product Details;
CasNo: 366-18-7
Molecular Formula: C10H8N2
Appearance: White crystalline powder
Good Manufacturer Fast Delivery 2,2'-Bipyridine 366-18-7 In Medicine
- Molecular Formula:C10H8N2
- Molecular Weight:157.195
- Appearance/Colour:White crystalline powder
- Vapor Pressure:0.584Pa at 25℃
- Melting Point:70-73 °C(lit.)
- Refractive Index:1.4820 (estimate)
- Boiling Point:272.499 °C at 760 mmHg
- PKA:pK1:-0.52(+2);pK2:4.352(+1) (20°C)
- Flash Point:107.243 °C
- PSA:25.78000
- Density:1.106 g/cm3
- LogP:2.14360
2,2'-Bipyridine(Cas 366-18-7) Usage
|
Chemical Properties |
White to light red crystalline powder. Soluble in ethanol, ether, benzene, chloroform and petroleum ether, 1 part of this product is about 200 parts of water. |
|
Uses |
2,2'-Bipyridine is a symmetrical bipyridine compound frequently used as a neutral ligand for forming complexes with metal ions. This molecule has a planar structure with a trans-conformation and crystallizes in the monoclinic crystal system. It plays a role as both a ferroptosis inhibitor and a chelating agent. The synthesis of 2,2'-bipyridine involves the reaction of pyridine with ferric chloride, where anhydrous pyridine and anhydrous ferric chloride are heated together at 300°C in a sealed tube. The resulting red-black crystals are then dissolved in hot water, impurities are removed with ether extraction, and excess pyridine is eliminated through steam heating. After making the mixture strongly basic, 2,2'-bipyridine is distilled off with steam. This compound can be further purified through various methods, such as crystallization from solvents like hexane or ethanol, precipitation from concentrated ethanol solutions with the addition of water, and chromatography on alumina or sublimation. Its UV absorption maximum occurs at 280nm in ethanol. |
|
Biochem/physiol Actions |
Metalloprotease inhibitor, high-affinity chelator of iron; may inhibit Fe2+ containing enzymes at 10?8?M. |
|
Safety Profile |
Poison by ingestion,subcutaneous, and intraperitoneal routes. Experimentalteratogenic data. Questionable carcinogen withexperimental tumorigenic data. Mutation data reported.When heated to decomposition it emits toxic fumes ofNOx. |
|
Consumer Uses |
ECHA has no public registered data indicating whether or in which chemical products the substance might be used. ECHA has no public registered data on the routes by which this substance is most likely to be released to the environment. |
366-18-7 Relevant articles
INVESTIGATION OF THE INTERACTION OF PYRIDINE WITH THE SURFACE OF LAMINAR SILICATES BY THE METHOD OF OPTICAL ELECTRONIC SPECTROSCOPY.
Sivalov,Tarasevich
, p. 214 - 218 (1981)
A study of the nature of the active site...
Bipyridine: The Most Widely Used Ligand. A Review of Molecules Comprising at Least Two 2,2‘-Bipyridine Units
Christian Kaes, Alexander Katz, and Mir Wais Hosseini
, Chem. Rev. 2000, 100, 10, 3553–3590
2,2‘-bipyridine unit has been used in a variety of approaches dealing with structural coordination chemistry and/or functional systems based on 2,2‘-bipyridine … by 2,2‘-bipyridine ligands …
Pd-catalyzed oxidative homocoupling of arylboronic acids in WEPA: A sustainable access to symmetrical biaryls under added base and ligand-free ambient conditions
Appa, Rama Moorthy,Lakshmidevi, Jangam,Naidu, Bandameeda Ramesh,Venkateswarlu, Katta
, (2021/01/11)
Symmetrical and unsymmetrical biaryls co...
366-18-7 Process route
-
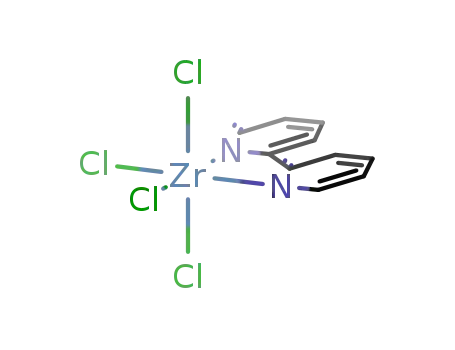
- 17099-99-9
tetrachloro-2,2'-bipyridylzirconium

-
![[2,2]bipyridinyl](/upload/2023/8/53fc9d45-670c-487b-ac27-ba0ca03de0b6.png)
- 366-18-7
[2,2]bipyridinyl

-
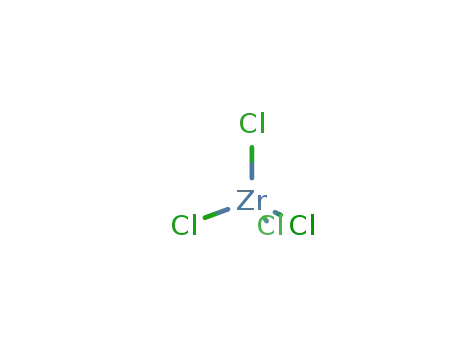
- 10026-11-6
zirconium(IV) chloride
| Conditions | Yield |
|---|---|
|
In neat (no solvent); heating under N2 above 400 °C;
|
-
![[Mn4(μ-O)6(2,2'-bipyridine)6][ClO4]4*H2O](/upload/2023/8/61d8cc45-745a-4dee-83fc-57f7d47e4522.png)
-
[Mn4(μ-O)6(2,2'-bipyridine)6][ClO4]4*H2O

-
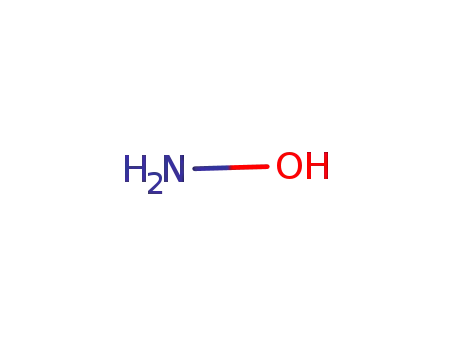
- 7803-49-8
hydroxylamine

-
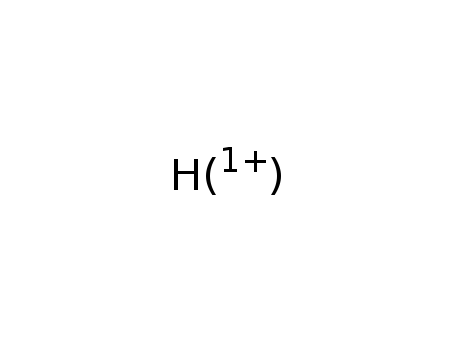
-
hydrogen cation

-
![[2,2]bipyridinyl](/upload/2023/8/53fc9d45-670c-487b-ac27-ba0ca03de0b6.png)
- 366-18-7
[2,2]bipyridinyl

-
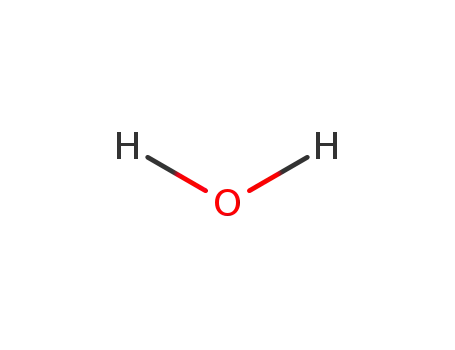
- 7732-18-5
water

-
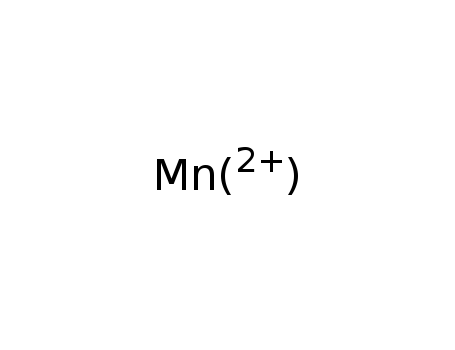
- 16397-91-4
manganese(II)

-
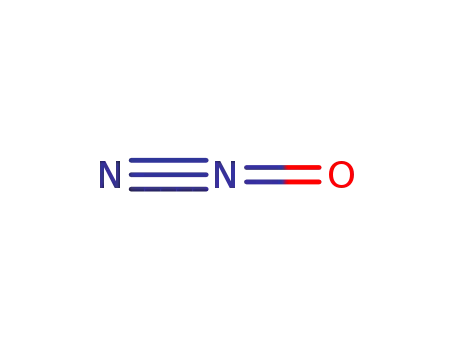
- 10024-97-2
dinitrogen monoxide
| Conditions | Yield |
|---|---|
|
With [2,2]bipyridinyl; In water; hydroxylamine oxidized by Mn complex in H2O at 25°C in the presence of 2,2'-bipyridine (mechanism discussed); not isolated, detcd. by UV; Kinetics;
|
|
|
With [2,2]bipyridinyl; In water-d2; hydroxylamine oxidized by Mn complex in D2O at 25°C in the presence of 2,2'-bipyridine (mechanism discussed); not isolated, detcd. by UV; Kinetics;
|
Relevant Products
-
Tropinone
CAS:532-24-1
-
Testosterone enanthate
CAS:315-37-7
-
4-Amino-3,5-dichloroacetophenone
CAS:37148-48-4