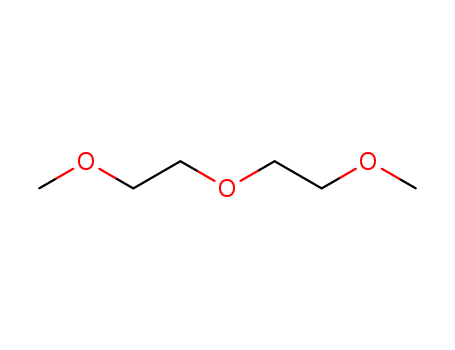
111-96-6
- Product Name:2-Methoxyethyl ether
- Molecular Formula:C6H14O3
- Purity:99%
- Molecular Weight:134.175
Product Details;
CasNo: 111-96-6
Molecular Formula: C6H14O3
Appearance: clear liquid
Global Trade High Purity 2-Methoxyethyl ether 111-96-6 In Bulk Supply
- Molecular Formula:C6H14O3
- Molecular Weight:134.175
- Appearance/Colour:clear liquid
- Vapor Pressure:3 mm Hg ( 20 °C)
- Melting Point:-64 ºC
- Refractive Index:1.4097
- Boiling Point:159.8 ºC at 760 mmHg
- Flash Point:70 ºC
- PSA:27.69000
- Density:0.914 g/cm3
- LogP:0.29580
2-Methoxyethyl ether (Cas 111-96-6) Usage
|
Description |
2-Methoxyethyl ether is a versatile chemical compound with applications as a solvent and in various chemical reactions, including its use in the synthesis of quinazolinones. It has also been the subject of research in terms of its solubility properties and interactions with different salts. |
|
Uses |
2-Methoxyethyl ether is widely used as a solvent in different industries due to its ability to dissolve various substances. It is a clear liquid at room temperature and is commonly employed for its solvent properties. Solvent; it is used as reaction medium for Grignard and similar synthesis. It has been used in experimental solubility studies with various binary mixtures and mathematical representations like the combined nearly ideal binary solvent (NIBS)/Redlich−Kister equation and modified Wilson model. It can be involved in various chemical reactions, such as intramolecular acceptorless dehydrogenative coupling, leading to the construction of structurally diverse quinazolinones. This reaction often involves the use of molecular oxygen as an oxidizing agent. |
|
Definition |
ChEBI: A polyether that is the dimethyl ether derivative of diethylene glycol. |
InChI:InChI:1S/C6H14O3/c1-7-3-5-9-6-4-8-2/h3-6H2,1-2H3
111-96-6 Relevant articles
Solubility of anthracene in binary alkane+ 2-methoxyethyl ether solvent mixtures
KAREN S. COYM,LINDSAY E. ROY,CARMEN E. HERNANDEZ &WILLIAM E. ACREE JR
, Chemical Engineering Communications Volume 162, 1997 - Issue 1
Experimental solubilities are reported for anthracene dissolved in six binary mixtures containing 2-methoxyethyl ether with n-hexane, n-heptane, n-octane, cyclohexane, methyl-cyclohexane and 2,2,4-trimethylpentane at 298.15 K.
Tetracarbonylcyclopentadienyl compounds of the group V transition metals
Werner, Robert P. M.,Filbey, Allen H.,Manastyrskyj, Switlana A.
, p. 298 - 300 (1964)
-
Excess molar enthalpies of (carbon dioxide+ethylene glycol dimethyl ether or 2-methoxyethyl ether) at the temperatures 298.15 K and 308.15 K and pressures from 7.5 MPa to 12.5 MPa
Jian Ping Zhao, Peter R. Tremaine
, The Journal of Chemical Thermodynamics Volume 28, Issue 6, June 1996, Pages 577-587
In contrast to the behaviour of ethylene glycol dimethyl ether mixtures, for which (∂HmE/∂p)Txis positive, the pressure dependence ofHmEfor the 2-methoxyethyl ether mixtures became negative in the solvent-rich region atT= 298.15 K. The Peng-Robinson equation reproduces the main features observed, but fails to predict the reversal in (∂HmE/∂p)Tx.
111-96-6 Upstream products
-
124-41-4
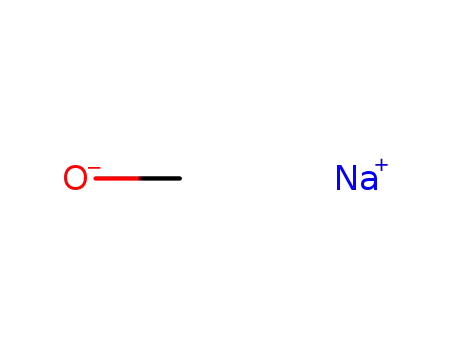
sodium methylate
-
111-44-4
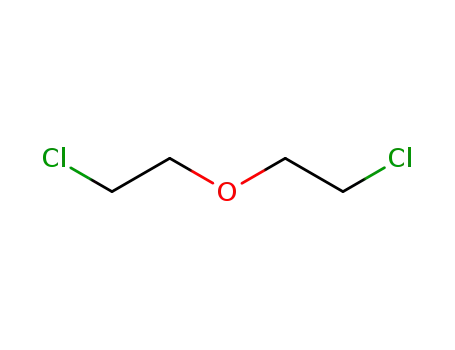
3-oxa-1,5-dichloropentane
-
109-86-4
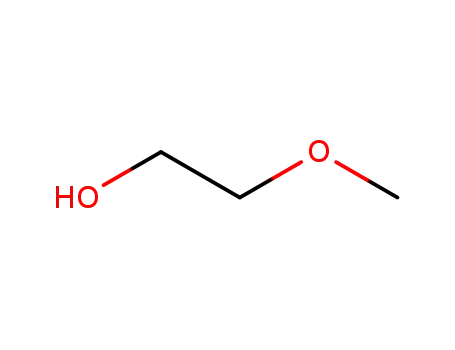
2-methoxy-ethanol
-
74-88-4
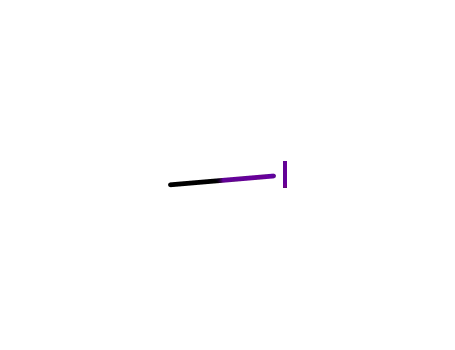
methyl iodide
111-96-6 Downstream products
-
66-25-1
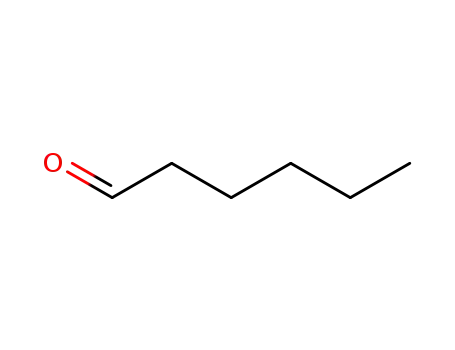
hexanal
-
100-52-7
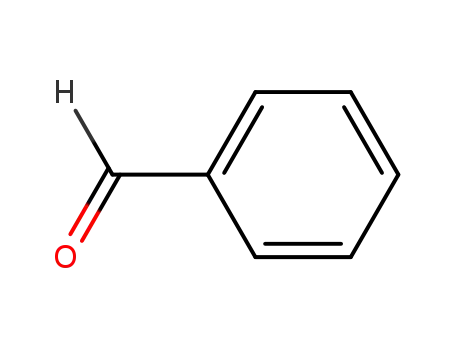
benzaldehyde
-
557-75-5
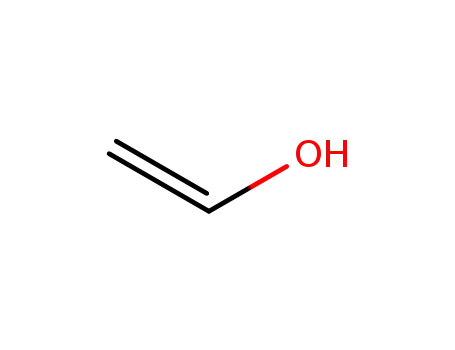
ethenol
-
926-02-3
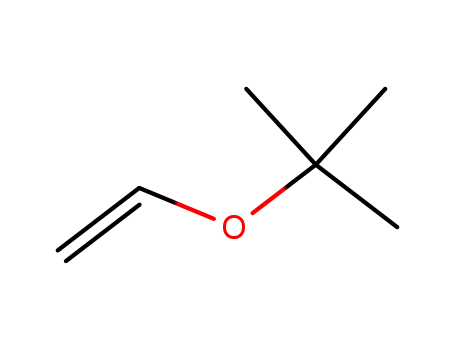
tert-butyl vinyl ether
Relevant Products
-
Isomaltitol
CAS:534-73-6
-
MK-677
CAS:159752-10-0
-
L-Valine
CAS:72-18-4